|
|
| | curium Chemical Properties |
| Melting point | 1345° | | Boiling point | bp (calc) 3110° | | density | 13.51; d 12.9 | | form | silvery metal | | color | silvery metal; hexagonal, hexane or
cubic | | EPA Substance Registry System | Curium (7440-51-9) |
| | curium Usage And Synthesis |
| General Description | Curium was discovered by Seaborg, James, and Ghiorso in 1944 during chemical fractionation of plutonium irradiated with alpha particles (32 MeV). The element was isolated in hydroxide form by Werner and Perlman in 1947 in microgram amounts, and later in 1950 by Crane, Wallmann, and Cunningham in elemental form. Crane et al. also studied its magnetic susceptibility and assigned 5f7 electron configuration to this element, analogous to 4f7 configuration of the element gadolinium in the lanthanide series. This man-made element was named curium in honor of Marie and Pierre Curie.
Curium does not occur in nature. Even if it had occured in the primordial age of earth, its longest lasting isoptope, Cm-247 (half-life of 17 million years),would almost have fully disintegrated during the more than three billion years of earth’s existence.
The element does not have any important commercial applications. Its isotopes Cm-242 and Cm-244 have potential applications to generate thermoelectric power for operation of instruments in space ships.
| | Production | Curium can be synthesized in a nuclear reactor by several methods. The first synthesis involved alpha particle bombardment of plutonium-239:

It may be synthesized by several other methods. Curium isotopes of lower mass numbers may be obtained by charged particle bombardments of plutonium239:
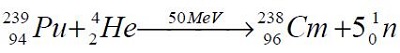
Curium-244 isotope may be obtained by irradiation of plutonium-239 by thermal neutrons:


Curium-242 isotope may be obtained in the same way from plutonium-239 by successive neutron capture and β¯ decay:

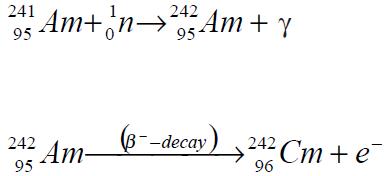
Also, higher isotopes of curium may be produced from curium-242 by neutron capture reactions.
The heavier isotopes of the element may result from rapid neutron capture process caused by intense neutron fluxes from thermonuclear explosions, followed by a series of β–decay
 | | Chemical Properties | silvery white, hard brittle metal; chemistry of trivalent state similar to that of trivalent lanthanides; α-emitter; hexagonal, a=0.3496nm, c=1.1331 nm; enthalpy of vaporization 1340 kJ/mol; ionic radius of Cm+++ is 0.0970nm; discovered in 1944; used in generating thermoelectric power for remote locations and in space; β-Cm is fcc, which is stable at <1340°C [HAW93] [MER06] [KIR78] | | Physical properties | After the discovery of plutonium and before elements 95 and 96 were discovered, theirexistence and properties were predicted. Additionally, chemical and physical properties werepredicted to be homologous (similar) to europium (63Eu) and gadolinium (64Gd), locatedin the rare-earth lanthanide series just above americium (95Am) and curium (96Cm) on theperiodic table. Once discovered, it was determined that curium is a silvery-white, heavymetal that is chemically more reactive than americium with properties similar to uraniumand plutonium. Its melting point is 1,345°C, its boiling point is ~1,300°C, and its density is13.51g/cm3. | | Isotopes | There are 23 isotopes of curium. All of them are man-made and radioactive.The most stable is curium-247, with a half-life of 1.56×10+7years (156,600,000 years),which through alpha decay transmutates into plutonium-243. | | Origin of Name | Named after Pierre and Marie Curie. | | Occurrence | There is no natural curium on Earth. All of its isotopes are man-made and artificiallyproduced through nuclear reactions with other elements. The curium isotope Cm-242 wasfirst produced by bombarding plutonium-239 with helium nuclei (alpha particles), whichcontributed neutrons that changed 94Pu to 96Cm. | | History | Although
curium follows americium in the periodic system, it was actually
known before americium and was the third transuranium
element to be discovered. Curium was identified by Seaborg, James,
4-10 The Elements
and Ghiorso in 1944 at the wartime Metallurgical Laboratory
in Chicago as a result of helium-ion bombardment of 239Pu in
the Berkeley, California, 60-inch cyclotron. Visible amounts
(30 μg) of 242Cm, in the form of the hydroxide, were first isolated
by Werner and Perlman of the University of California in
1947. In 1950, Crane, Wallmann, and Cunningham found that
the magnetic susceptibility of microgram samples of CmF3 was
of the same magnitude as that of GdF3. This provided direct
experimental evidence for assigning an electronic configuration
to Cm+3. In 1951, the same workers prepared curium in
its elemental form for the first time. Sixteen isotopes of curium
are now known. The most stable, 247Cm, with a half-life
of 16 million years, is so short compared to the Earth’s age that
any primordial curium must have disappeared long ago from
the natural scene. Minute amounts of curium probably exist
in natural deposits of uranium, as a result of a sequence of
neutron captures and β decays sustained by the very low flux
of neutrons naturally present in uranium ores. The presence
of natural curium, however, has never been detected. 242Cm
and 244Cm are available in multigram quantities. 248Cm has
been produced only in milligram amounts. Curium is similar
in some regards to gadolinium, its rare-earth homolog, but it
has a more complex crystal structure. Curium is silver in color,
is chemically reactive, and is more electropositive than aluminum.
CmO2, Cm2O3, CmF3, CmF4, CmCl3, CmBr3, and CmI3
have been prepared. Most compounds of trivalent curium are
faintly yellow in color. 242Cm generates about three watts of
thermal energy per gram. This compares to one-half watt per
gram of 238Pu. This suggests use for curium as a power source.
244Cm is now offered for sale by the O.R.N.L. at $185/mg plus
packing charges. 248Cm is available at a cost of $160/μg, plus
packing charges, from the O.R.N.L. Curium absorbed into the
body accumulates in the bones, and is therefore very toxic as
its radiation destroys the red-cell forming mechanism. The
maximum permissible total body burden of 244Cm (soluble) in
a human being is 0.3 μCi (microcurie). | | Characteristics | Curium is a synthetic (not natural) transuranic element of the actinide series. It was determinedthat curium’s major valence and oxidation state was +3, similar to other elements of thisseries. The most stable isotope of curium is curium-247, with a half-life of 1.56×10+7years. | | Uses | There are no major commercial uses for curium because of the extremely small amountproduced. In the future, the most important use of curium may be to provide the power forsmall, compact thermoelectric sources of electricity, by generating heat through the nucleardecay of radioisotope curium-241. These small, efficient power sources can be used in individualhomes or remote regions to provide electricity to areas that cannot secure it from othersources. It could also be used as a source of electricity in spacecraft. However, today curium’smain use is for basic scientific laboratory research. | | Uses | 242Cm and 244Cm as power sources in radionuclide batteries for space and medical applications. 242Cm as radioactive heat source. 248Cm in accelerator studies to form superheavy elements. | | Definition | curium: Symbol Cm. A radioactivemetallic transuranic element belongingto the actinoids; a.n. 96; massnumber of the most stable isotope247 (half-life 1.64 × 107 years); r.d.(calculated) 13.51; m.p. 1340±40°C.There are nine known isotopes. Theelement was first identified by GlennSeaborg (1912–99) and associates in1944 and first produced by L. B.Werner and I. Perlman in 1947 bybombarding americium–241 withneutrons. | | Definition | A highly toxic radioactive
silvery element of the actinoid series
of metals. A transuranic element, it is
not found naturally on Earth but is synthesized
from plutonium. Curium-244 and
curium-242 have been used in thermoelectric
power generators. | | Hazard | Curium metal and its compounds are radioactive bone-seeking poisons that attack theskeletal system of humans and animals. Care must be used in handling them. |
| | curium Preparation Products And Raw materials |
|