- METHYL FLUORIDE
-

- $1.00 / 1kg
-
2019-07-06
- CAS:593-53-3
- Min. Order: 1kg
- Purity: 95%-99%
- Supply Ability: 100kg
Related articles - What is Methyl Fluoride?
- Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable....
- Sep 16,2020
|
| | METHYL FLUORIDE Basic information |
| Product Name: | METHYL FLUORIDE | | Synonyms: | METHYL FLUORIDE;CARBON MONOFLUORIDE;CARBON MONOFLUORIDE POLYMER;FLUOROMETHANE;FC-41;HALOCARBON 41;Fron-41;Fluoromethane, 99+% | | CAS: | 593-53-3 | | MF: | CH3F | | MW: | 34.03 | | EINECS: | 209-796-6 | | Product Categories: | refrigerants | | Mol File: | 593-53-3.mol | 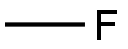 |
| | METHYL FLUORIDE Chemical Properties |
| Melting point | -115°C | | Boiling point | -79°C | | density | 0,877 g/cm3 | | vapor pressure | 1965-5874kPa at 0-15℃ | | refractive index | 1.1740 | | Water Solubility | 1.787g/L(29.9 ºC) | | Stability: | Stable. Extremely flammable. Incompatible with strong oxidizing agents. | | LogP | 0.51 at 20℃ and pH7 | | CAS DataBase Reference | 593-53-3(CAS DataBase Reference) | | EPA Substance Registry System | HFC-41 (593-53-3) |
| Hazard Codes | F,F+ | | Risk Statements | 12 | | Safety Statements | 16-33 | | RIDADR | 2454 | | Hazard Note | Flammable | | HazardClass | 2.1 | | HS Code | 2903430010 |
| | METHYL FLUORIDE Usage And Synthesis |
| Chemical Properties | colourless gas with an ether-like odour | | Definition | ChEBI: A member of the class of fluoromethanes that is methane in which a single hydrogen is substituted by a fluorine atom. | | General Description | METHYL FLUORIDE (or fluoromethane) is a colorless flammable gas which is heavier than air. METHYL FLUORIDE has an agreeable ether-like odor. METHYL FLUORIDE is narcotic in high concentrations. METHYL FLUORIDE burns with evolution of hydrogen fluoride. The flame is colorless, similar to alcohol. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. | | Air & Water Reactions | Highly flammable. METHYL FLUORIDE burns in air with evolution of hydrogen fluoride. | | Reactivity Profile | Halogenated aliphatic compounds, such as METHYL FLUORIDE, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Low molecular weight haloalkanes are highly flammable and can react with some metals to form dangerous products. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. The prolonged mixing of halogenated solvents with metallic or other azides may cause the slow formation of explosive azides, for example methylene chloride and sodium azide, [Chem. Eng. News, 1986, 64(51)]. | | Hazard | Flammable. Narcotic in high concentrations. | | Health Hazard | Vapors may cause dizziness or asphyxiation without warning. Some may be irritating if inhaled at high concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases. | | Fire Hazard | EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. | | Safety Profile | Narcotic in high
concentrations. Acts as a simple asphyxiant.
Burns with evolution of hydrogen fluoride.
The flame is about as colorless as that of
alcohol. When heated to decomposition it
emits toxic fumes of F-. |
| | METHYL FLUORIDE Preparation Products And Raw materials |
| Raw materials | Ethylfluorodimethylsilane-->1-Pentene-1,3-dione, 4,4,5,5,5-pentafluoro-2-(trifluoromethyl)--->3-Pentanone, 1,1,1,2,2,5,5,5-octafluoro-4-(trifluoromethyl)--->Propanoyl fluoride, 2,3,3,3-tetrafluoro--->Propane, 1,1,2,3,3-pentafluoro-1,3-dimethoxy--->(Fluoromethyl)trimethylsilane-->1,1,2,3,3,3-HEXAFLUOROPROPYL METHYL ETHER-->(METHOXYMETHYL)TRIMETHYLSILANE-->3,3,3-Trifluoro-2-(trifluoromethyl)propionyl fluoride-->1,1,3,3,3-Pentafluoro-2-trifluoromethylpropyl methyl ether-->Dimethyl ether-->Trimethylfluorosilane | | Preparation Products | 2,4-Dimethylbromobenzene-->4-Fluoro-2-methylaniline-->3-Fluoro-2-methylaniline-->1-(1-METHYL-1H-INDOL-3-YL)-1-ETHANONE-->Pentafluoroethane-->Tetrafluoroethylene |
|