| Company Name: |
Daicel Chiral Technologies (China)CO.,LTD
|
| Tel: |
021-50460086-9 15921403865 |
| Email: |
han_yajun@dctc.daicel.com |
| Products Intro: |
Product Name:1,1,1-Trifluoro-methanol-1-benzoate
CAS:1035797-66-0
Purity:95%/98% Package:1G;100G; 1KG Remarks:201221
|
| Company Name: |
JinOu Biomedical (Nanjing) Co., Ltd.
|
| Tel: |
13000000000 |
| Email: |
jinoupharma@163.com |
| Products Intro: |
Product Name:trifluoromethyl benzoate
CAS:1035797-66-0
Purity:99% HPLC Package:100G;1KG;100KG
|
| Company Name: |
Ningbo Chemrio Chemtech Co,.Ltd.
|
| Tel: |
0574-27891269 18667892864 |
| Email: |
frank.xiaodong@chemriotech.com |
| Products Intro: |
Product Name:Trifluoromethyl benzoate
CAS:1035797-66-0
Purity:98% Package:25KG;5KG;1KG
|
|
| | trifluoromethyl benzoate Basic information |
| | trifluoromethyl benzoate Chemical Properties |
| Boiling point | 245.4±40.0 °C(Predicted) | | density | 1.322±0.06 g/cm3(Predicted) |
| | trifluoromethyl benzoate Usage And Synthesis |
| Chemical Properties | Trifluoromethyl benzoate (TFBz) is developed as a new shelf-stable trifluoromethoxylation reagent, which can be easily prepared from inexpensive starting materials using KF as the only fluorine source.
| | Preparation | 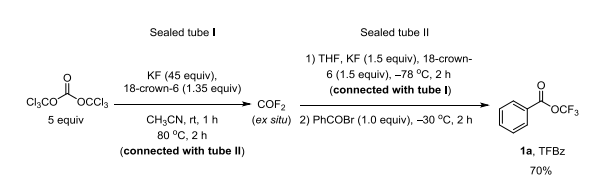
To a 350-mL oven-dried sealed tube (I) equipped with a robust stir bar were added
KF (65.4 g, 1125 mmol, 45 equiv) and 18-crown-6 (8.9 g, 33.75 mmol, 1.35 equiv) in
the glove box. Tube (I) was removed out of glove box and 230 mL dry CH3CN was
added under N2. To another 350-mL oven-dried sealed tube (II) equipped with a
robust stir bar were added dried KF (2.18 g, 37.5 mmol, 1.5 equiv) and dried
18-crown-6 (9.91 g, 37.5 mmol, 1.5 equiv) in the glove box. Tube (II) was removed
out of glove box and 100 mL dry THF was added under N2. Tube (I) was placed into
dry-ice/acetone, and triphosgene (37.1 g, 125 mmol, 5 equiv) was added until the
solution was frozen. Tube (I) was sealed. The mixture was warmed to room
temperature and stirred for 1 h. Tube (II) was evacuated for 1-2 seconds before it was
connected with tube (I) through a gas-tube and tube (I) was heated to 80 oC while tube
(II) was cooled to -78 oC. The COF2 was transferred from tube (I) to tube (II) for 2 h.
Then benzoyl bromide (4.63 g, 25 mmol, 1.0 equiv) was added to tube (II) at -78 oC
under N2, then the solution was warmed to -30 oC and stirred for 2 h while the
solution became very stick. Then soduim trifluoromethanesulfonate (8.6 g) was added
at -78 oC under N2 and stirred for another 30 min at -30 oC. Tube (II) was warmed to
room temperature and over-excess COF2 was absorbed by 10% NaOH (aq) carefully.
The difluorophosgene dissolved in the reaction mixture in tube (II) was also
completely removed by flowing N2 gas to the reaction mixture and absorbed by 10%
NaOH (aq). The solvent was evaporated under vacuum. Et2O was added and the mixture was filtered through a pad of celite. The solvent was evaporated under
vacuum and product was further purified by flash column chromatography using
petroleum ether (30-60 oC) as eluent to give product 1a 3.31g as a colorless liquid.
(Caution! COF2 was a toxic gas, so it should be handled in a well ventilated hood!)
TFBz has been stored in the air at room temperature for 5 months without detectable
decomposition. Moreover, a mixture of TFBz and water (1:2, v/v) can be stored at
room temperature for at least 5 days without detectable decomposition. [1]Colorless liquid. 1H NMR (400 MHz, CDCl3) δ 8.08 (dd, J = 8.3, 1.2 Hz, 2H), 7.70 (t,
J = 7.5 Hz, 1H), 7.53 (t, J = 7.9 Hz, 2H).
19F NMR (376 MHz, CDCl3) δ -57.71 (s,
3F). 13C NMR (126 MHz, CDCl3) δ 159.0, 135.1, 130.5, 128.9, 126.6 (q, J = 1.6 Hz),
119.9 (q, J = 265.4 Hz). MS (EI, m/z, %): 190 (M+
, 22), 105 (100), 77 (58); HRMS
(EI) (m/z): [M]+ Calcd for C8H5F3O2, 190.0242; found, 190.0247.
| | References | [1] MIN ZHOU. Trifluoromethyl Benzoate: A Versatile Trifluoromethoxylation Reagent[J]. Journal of the American Chemical Society, 2018, 140 22: 6801-6805. DOI:10.1021/jacs.8b04000. |
| | trifluoromethyl benzoate Preparation Products And Raw materials |
|