|
ChemicalBook Optimization Suppliers |
|
| 化学名: | 炭酸カリウム | | 英語化学名: | Potassium carbonate | | 别名: | POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,FCC;POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,PURIFIED;POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,REAGENT,ACS;POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,REAGENT,ACS(BULK;POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,USP;POTASSIUM CARBONATE: 99.998% PURATREM;Potassium carbonate,99+%,for analysis,anhydrous;Potassium Carbonate, Puratronic (Metals Basis) | | CAS番号: | 584-08-7 | | 分子式: | K2CO3 | | 分子量: | 138.21 | | EINECS: | 209-529-3 | | カテゴリ情報: | Chemical Synthesis;Inorganic Bases;Materials Science;Metal and Ceramic Science;Potassium Salts;Synthetic Reagents;Inorganics;metal carbonate;FOOD ADDITIVES;Chemical Synthesis;Inorganic BasesMetal and Ceramic Science;Potassium Salts;Salts;Synthetic Reagents;INORGANIC & ORGANIC CHEMICALS;Analytical Reagents for General Use;O-P, Puriss p.a. ACS;Puriss p.a. ACS;O-P, Puriss p.a.;Puriss p.a.;Reagent Plus;AlphabeticalChemical Synthesis;Biological Buffers;BioUltra Buffers;Inorganic Bases;Reagent Grade;Pharmacopoeia (USP);Pharmacopoeia A-ZPharmacopoeia (USP);Pharmacopoeial OrganicsEssential Chemicals;Routine Reagents;USP;ACS GradeEssential Chemicals;Adsorbents, Filter Aids and Drying Agents;Essential Chemicals;Other Drying Agents;584-08-7 | | Mol File: | 584-08-7.mol | 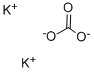 |
| 融点 | 891 °C (lit.) | | 沸点 | decomposes [STR93] | | 比重(密度) | 2.43 g/mL at 25 °C | | かさ密度 | 750kg/m3 | | 貯蔵温度 | Store at +5°C to +30°C. | | 溶解性 | H2O: 1 M at 20 °C, clear, colorless | | 酸解離定数(Pka) | 10.33[at 20 ℃] | | 外見 | powder | | 色 | Yellow | | 比重 | 2.29 | | PH | 10.52(1 mM solution);11(10 mM solution);11.36(100 mM solution); | | 臭い (Odor) | at 100.00?%. odorless | | 水溶解度 | 1120 g/L (20 ºC) | | Sensitive | Hygroscopic | | 極大吸収波長 (λmax) | λ: 260 nm Amax: 0.03
λ: 280 nm Amax: 0.02 | | Merck | 14,7619 | | BRN | 4267587 | | Dielectric constant | 5.6(16.0℃) | | 安定性: | Stable. Incompatible with moisture, acids, magnesium bromine trifluoride and magnesium bromine trichloride. | | CAS データベース | 584-08-7(CAS DataBase Reference) | | NISTの化学物質情報 | Dipotassium carbonate(584-08-7) | | EPAの化学物質情報 | Potassium carbonate (584-08-7) |
| | 炭酸カリウム Usage And Synthesis |
| 外観 | 白色の粉末又は粒 | | 定義 | 本品は、炭酸の二カリウム塩であり、次の化学式で表される。 | | 溶解性 | 水に溶けやすく、エタノールにほとんど溶けない。 | | 解説 | 炭酸カリウム,たんさんかりうむ,K2CO3(138.21).単にカリともいう.水酸化カリウム水溶液に二酸化炭素を作用させて濃縮すると得られる.また,炭酸水素カリウムの熱分解により無水物が得られる.無水物は白色の粉末.密度2.42 g cm-3.融点891 ℃(分解).潮解性で,水に易溶,エタノール,アセトンに不溶.水溶液はアルカリ性を示し,二酸化炭素を吸収して炭酸水素カリウムを生じる.水溶液からは常温で二水和物が析出する.ほかに1.5水和物(単斜晶系.密度2.04 g cm-3)がある.軟せっけん,ガラス,陶磁器,医薬品などの原料,染色,漂白,脱水剤,肥料,炭酸ナトリウムと混合して分析用融剤などに用いられる. | | 用途 | 石鹸、ガラス、陶器、赤色顔料、各種カリウム塩の製造、; エッティング、リソグラフ、皮のなめしや仕上げ等の工程、 シャンプー、分析化学で有機溶剤の脱水、医薬品助剤(アルカリ化剤) | | 用途 | 高純度金属化合物。 | | 用途 | 食品添加物用。 | | 化粧品の成分用途 | pH調整剤 | | 効能 | アルカリ化剤 | | 使用上の注意 | 吸湿性が強い | | 化学的特性 | Potassium carbonate is a white, crystalline, salt that forms basic aqueous solutions used in the production of fertilizer, glass, ceramics, explosives, soaps, chemicals, and wool treatments. It was the main compound once referred to as potash, although the term today is not reserved exclusively for potassium carbonate, but for several potassium salts. In the fertilizer industry, potash refers to potassium oxide, K2O, rather than potassium carbonate. Pearlash is a purer form of potash made by heating potash to remove impurities. | | 使用 | Potassium carbonate is used in the chemical industry as a source of inorganic potassium salts (potassium silicates, potassium bicarbonate), which are used in fertilizers, soaps, adhesives, dehydrating agents, dyes, and pharmaceuticals. Potassium carbonate used to make potassium lye produces soft soaps, which are liquids or semisolids rather than solids. Other uses of potassium carbonate includes use as a fire suppressant in extinguishers, as a CO2 absorbent for chemical processes and pollution control, an antioxidant in rubber additives, and in pharmaceutical formulations. | | 調製方法 | Potassium carbonate can be obtained from ash or by electrolysis of potassium chloride. Another method involves reacting potassium hydroxide with carbon dioxide. The resulting liquid carbonate contains approximately 50% potassium carbonate in water. However, the solid product, which contains over 70% potassium carbonate, is expensive and only suitable for certain types of acidic soil. Additionally, neutralizing caustic potash with carbon dioxide also yields potassium carbonate. | | 定義 | ChEBI: Potassium carbonate is a potassium salt that is the dipotassium salt of carbonic acid. It has a role as a catalyst, a fertilizer and a flame retardant. It is a carbonate salt and a potassium salt. | | 製造 | 炭酸のカリウム塩。水酸化カリウムの45~50%溶液に二酸化炭素を吹き込み、全部が炭酸カリウムとなったところで不純物を濾別(ろべつ)し、真空濃縮することによって製造される。 2KOH+CO2→K2CO3+H2O また、炭酸水素カリウムとして析出させてから熱分解する方法も行われる。 2KHCO3→K2CO3+CO2+H2O | | 一般的な説明 | Potassium carbonate (K2CO3) is a white salt, soluble in water (insoluble in ethanol) which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It presents a large capacity to absorb moisture.Corrosive to metals and tissue. Density 12.8 lb /gal. Used to make soaps, other potassium compounds, in liquid fertilizers. | | 空気と水の反応 | Water soluble. Addition of water evolves heat. | | 反応プロフィール | Potassium carbonate neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose, to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995]. | | 危険性 | Solutions irritating to tissue. | | 健康ハザード | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | 火災危険 | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. | | 农业用途 | Potassium carbonate is used in agriculture and food production. Potassium carbonate is used as a spray or drip fertilizer and also as a constituent of compound fertilizers. Its high water solubility and alkaline property make it useful for supplying potassium to acidic soils, especially in vineyards and orchards. Dutch-processed cocoa uses potassium carbonate as an alkalizing agent to neutralize the natural acidity of cocoa. It is used to produce food additives like potassium sorbate and monopotassium phosphate. | | 工業用途 | Potassium carbonate is used for numerous applications. Its primary use is in the production of specialty glasses and ceramics. It is used to make optical glass, glass used for video screens in televisions and computers, and laboratory glassware. Its is used in certain glasses rather then cheaper sodium carbonate owing to its better compatibility with lead, barium, and strontium oxides incorporated in these glasses. These oxides lower the melting point of glass and produce a softer glass. Potassium carbonate has a higher refractive index than sodium carbonate producing a more brilliant glass. Potassium carbonate is a common flux combined with titanium dioxide to produce frits used in ceramics. A frit is a calcined mixture of fine silica, a pigment, and a flux that is ground a specific particle size and used to produce glazes, enamels, and additives in glass making. | | 生物活性 | Potassium carbonate(K2CO3) is used as a drying agent for ketones, alcohols, amines, and non-acidic compounds before distillation. It serves as a buffering agent and controls pH during wine and mead production. Potassium carbonate is also used in the extraction of artemisinin, a potent antimalarial drug, from Artemisia annua plant extracts. | | 安全性プロファイル | Poison by ingestion. A
strong caustic. Incompatible with KCO,
chlorine trifluoride, magnesium. Mutation
data reported. When heated to
decomposition it emits toxic fumes of K2O. | | 純化方法 | It crystallises from water between 100o and 0o. The solubility in H2O is 105% at 0o, 127% at 60o and 205% at 135o (b of saturated solution). After two recrystallisations of technical grade material, it had B, Li and Fe at 1.0, 0.04 and 0.01 ppm, respectvely. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963.] | | 参考文献 | https://en.wikipedia.org/wiki/Potassium_carbonate
https://www.drugs.com/inactive/potassium-carbonate-106.html
http://www.sciencelab.com/msds.php?msdsId=9926681
https://pubchem.ncbi.nlm.nih.gov/compound/Potassium-Carbonate
Solubility of Potassium Carbonate and Potassium Hydrocarbonate in Methanol DOI:10.1021/JE020012V |
|