| Company Name: |
J & K SCIENTIFIC LTD.
|
| Tel: |
010-82848833 400-666-7788 |
| Email: |
jkinfo@jkchemical.com |
| Products Intro: |
Product Name:PivaMpicillin Hydrochloride
CAS:26309-95-5
Package:100Mg,10Mg
|
| Company Name: |
Chemsky (shanghai) International Co.,Ltd
|
| Tel: |
021-50135380 |
| Email: |
shchemsky@sina.com |
| Products Intro: |
Product Name:PivaMpicillin Hydrochloride;(2S,5R,6R)-6-[[(2R)-2-AMino-2-phenylacetyl]aMino]-3,3-diMethyl-7-oxo-4-thia-
1-azabicyclo[3.2.0]heptane-2-carboxylic Acid (2,2-DiMethyl-1-oxopropoxy)Methyl Ester Hydrochloride; Alphacilina; Alphacillin; AMpicillin PivaloxyoxyMethyl Ester Hydrochloride;
CAS:26309-95-5
Purity:98+% Package:1Mg;5Mg;10Mg;50Mg;100Mg;500Mg
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Email: |
marketing1@energy-chemical.com |
| Products Intro: |
Product Name:Pivampicillin Hydrochloride
CAS:26309-95-5
Purity:NULL Package:100mg;10mg Remarks:NULL
|
|
| Product Name: | Pivampicillin Hydrochloride | | Synonyms: | (pivaloyloxy)methyl [2S-[2alpha,5alpha,6beta(S*)]]-6-(2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate monohydrochloride;PIVAMPICILLINHYDROCHLORIDE;(2S,5R,6R)-6-[[(2R)-2-AMino-2-phenylacetyl]aMino]-3,3-diMethyl-7-oxo-4-thia-
1-azabicyclo[3.2.0]heptane-2-carboxylic Acid (2,2-DiMethyl-1-oxopropoxy)Methyl Ester Hydrochloride;Alphacilina;Alphacillin;AMpicillin PivaloxyoxyMethyl Ester Hydrochloride;6β-((R)-2-amino-2-phenyl-acetylamino)-penicillanic acid 2,2-dimethyl-propionyloxymethyl ester;Centurina | | CAS: | 26309-95-5 | | MF: | C22H30ClN3O6S | | MW: | 500.0081 | | EINECS: | 2476042 | | Product Categories: | Sulfur & Selenium Compounds;Pharmaceuticals;Intermediates & Fine Chemicals;Heterocycles;Chiral Reagents | | Mol File: | 26309-95-5.mol | 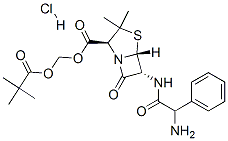 |
| | Pivampicillin Hydrochloride Chemical Properties |
| Melting point | 155-156° (decomp) | | alpha | D20 +196° (c = 1 in water) | | storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere | | solubility | DMSO (Slightly), Water (Slightly, Sonicated) | | pka | pKa ~7.0(at 25℃) | | form | Solid | | color | White |
| Toxicity | LD50 in mice, rats (g/kg): 3.34, 5.00 orally; 3.60, 4.50 s.c. (von Daehne) |
| | Pivampicillin Hydrochloride Usage And Synthesis |
| Description | Pivampicillin Hydrochloride is a pivaloyloxymethyl ester of ampicillin. It is a prodrug, which is thought to enhance the oral bioavailability of ampicillin because of its greater lipophilicity compared to that of ampicillin. | | Mechanism of action | Pivampicillin hydrochloride is not due to the drug itself but to pivalate, which is mostly removed from the body by forming a conjugate with carnitine. Although short-term use of these drugs can cause a marked decrease in blood levels of carnitine,it is unlikely to be of clinical significance;long-term use, however, is not recommended. | | Acute toxicity | Oral-rat LD50: 5000 mg/kg; Oral-mouse LD50: 3340 mg/kg. | | Originator | Maxifen ,Sharp and Dohme, W. Germany ,1972 | | Uses | Semi-synthetic antibiotic related to Penicillin (P223500). Antibacterial. | | Manufacturing Process | (A) Pivaloyloxymethyl D(-)-α-azidobenzylpenicillinate: To a suspension of potassium D(-)α-azidobenzylpenicillinate (4.14 g) and potassium dicarbonate(1.5 g) in acetone (100 ml) and 10% aqueous sodium iodide (2 ml), chloromethyl pivalate (2.7 ml) was added and the mixture refluxed for 2 hours. After cooling, the suspension was filtered and the filtrate evaporated to dryness in vacuo. The remaining residue was washed repeatedly by decantation with petroleum ether to remove unreacted chloromethyl pivalate. The oily residue was taken up in ethyl acetate (100 ml), and the resulting solution washed with aqueous sodium bicarbonate and water, dried and evaporated in vacuo to yield the desired compound as a yellowish gum, which crystallized from ether, melting point 114°C to 115°C.
(B) Pivaloyloxymethyl D(-)-α-aminobenzylpenicillinate, hydrochloride: To a solution of pivaloyloxymethyl D(-)-α-azidobenzylpenicillinate (prepared as described above) in ethyl acetate (75 ml) a 0.2 M phosphate buffer (pH 2.2) (75 ml) and 10% palladium on carbon catalyst (4 g) were added, and the mixture was shaken in a hydrogen atmosphere for 2 hours at room temperature. The catalyst was filtered off, washed with ethyl acetate (25 ml) and phosphate buffer (25 ml), and the phases of the filtrate were separated. The aqueous phase was washed with ether, neutralized (pH 6.5 to 7.0) with aqueous sodium bicarbonate, and extracted with ethyl acetate (2 x 75 ml). To the combined extracts, water (75 ml) was added, and the pH adjusted to 2.5 with 1 N hydrochloric acid. The aqueous layer was separated, the organic phase extracted with water (25 ml), and the combined extracts were washed with ether, and freeze-dried. The desired compound was obtained as a colorless, amorphous powder.
The purity of the compound was determined iodometrically to be 91%. A crystalline hydrochloride was obtained from isopropanol with a melting point of 155°C to 156°C (dec.). | | Therapeutic Function | Antibacterial | | Contact allergens | Pivampicillin is a prodrug of ampicillin. It caused sensitization
in 56 workers at a penicillin factory. Pivampicillin
and pivmecillinam were responsible for contact dermatitis
in pharmaceutical production workers. Ampicillin,
mecillinam or amdinocillin, penicillin V and penicillin
G were also implicated in cross-reactions. | | Safety Profile | Moderately toxic by ingestion andsubcutaneous routes. When heated todecomposition it emits very toxic fumes of NOx, SOx, andHCl. |
| | Pivampicillin Hydrochloride Preparation Products And Raw materials |
|