|
|
| | POLY(VINYLIDENE FLUORIDE-CO-HEXAFLUOROPROPYLENE) Basic information |
| Product Name: | POLY(VINYLIDENE FLUORIDE-CO-HEXAFLUOROPROPYLENE) | | Synonyms: | 1-Propene,1,1,2,3,3,3-hexafluoro-,polymerwith1,1-difluoroethene;POLY(VINYLIDENE FLUORIDE-CO-HEXAFLUOROPROPYLENE);POLY(VINYLIDENE FLUORIDE-CO-HEXAFLUORO-P ROPYLENE), MELT INDEX 4-10;POLY(VINYLIDENE FLUORIDE-CO-HEXAFLUORO-P ROPYLENE), AVERAGE MN CA. 130,000;POLY(VINYLIDENE FLUORIDE-CO-HEXAFLUORO-P ROPYLENE), AVERAGE MN CA. 110,000;HEXAFLUOROPROPYLENE-VINYLDENE-FLUORIDE,COPOLYMER;HEXAFLUOROPROPYLENE-VINYLIDENEFLUORIDE,COPOLYMER;Hexafluoropropylene-vinylidene fluoride: (Viton) | | CAS: | 9011-17-0 | | MF: | C5H2F8 | | MW: | 214.06 | | EINECS: | | | Product Categories: | Hydrophobic Polymers;Materials Science;Polymer Science;Fluorocarbons;Hydrophobic Polymers;Polymer Science;Polymers | | Mol File: | 9011-17-0.mol | 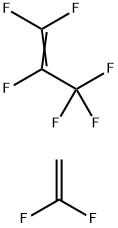 |
| | POLY(VINYLIDENE FLUORIDE-CO-HEXAFLUOROPROPYLENE) Chemical Properties |
| | POLY(VINYLIDENE FLUORIDE-CO-HEXAFLUOROPROPYLENE) Usage And Synthesis |
| Chemical Properties | A primary advantage of fluororubber is its exceptional heat,
chemical, and solvent resistance. The copolymer is biologically
inert. The decomposition products of FPM depend
on the chemical composition of the intact polymer and also
on the conditions under which it is decomposed. The temperature
to which the polymer is subjected, the atmosphere in
which decomposition occurs, and the material of the vessel
used can alter the kinds and quantities of the decomposition
products. When a copolymer of vinylidene fluoride and
hexafluoropropylene decomposed at 550 and 800°C, carbon
monoxide and carbon dioxide were produced. Hydrogen
fluorides and other fluorocarbons have been reported as
decomposition products. | | Uses | Wire and cable coatings, flexible and corrosion-resistant tubing, and liners for pipes and tanks. | | Uses | Details of the processes used to produce vinylidene fluoride-hexafluoro�propylene copolymers have not been disclosed but the copolymers may be prepared by emulsion polymerization under pressure using a persulphate�bisulphite initiator system. Highly fluorinated surfactants, such as am�monium perfluorooctoate, are most commonly used in order to avoid chain
transfer reactions. The preferred vinylidene fluoride content for commercial
copolymers is about 70% mole. The structure of such a copolymer might be
represented as follows:
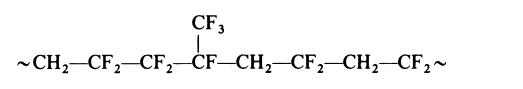
NMR studies confirm that head-to-tail arrangements predominate in the
copolymers. It may be noted that under the conditions normally used to
prepare commercial copolymers, hexafluoropropylene does not homopolymerize and so the molar proportion of this monomer in a copolymer cannot
exceed 50% whatever the composition of the monomer feed.
Commercial copolymers typically have an average molecular weight (Mn)
of about 70000.
Since the copolymers are saturated, they cannot be vulcanized by conventional sulphur systems. However, they may be vulcanized by aliphatic amines
and derivatives, aromatic dihydroxylic compounds and peroxides; all of these
methods are practised commercially. Free diamines react too quickly, caus�ing premature vulcanization or scorching during mixing. One method of
reducing the tendency to scorch is to use diamines in the form of their inner
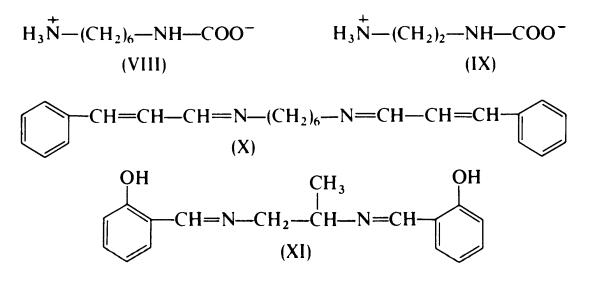
carbamates; hexamethylenediamine carbamate (VIII) and ethylenediamine
carbamate (IX) are employed commercially. Alternatively, the Schiff's bases
of diamines function as delayed-action vulcanizing agents; examples of
compounds of this type are N, N'-dicinnamylidene-l,6-hexanediamine (X)
and N, N'-disalicylpropylenediamine (XI).
 | | Preparation | The usual commercial practice is to mix the rubber on a mill with the
diamine derivative, together with filler (such as carbon black or silica) and a
metal oxide. The compounded stock is then given a short press cure (typically, 0.5 hours at 150??C) and a long oven cure (typically, 24 hours at 200??C).
The reactions which occur in the vulcanization of vinylidene fluoridehexafluoropropylene copolymers have been extensively studied and a
three-stage process is thought to be involved. The first step is postulated as
the elimination of hydrogen fluoride from the polymer upon treatment with a
base. Probably the tertiary fluorine atom of the hexafluoropropylene unit is
the most readily removed. The initially formed double bonds then activate
the elimination of hydrogen fluoride from neighbouring groups to give a
conjugated system:
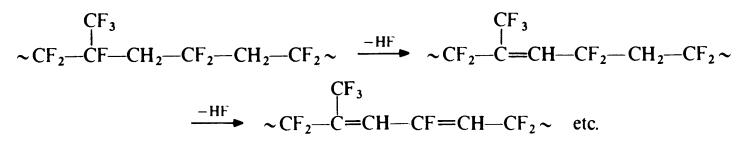
Elimination is thought to be catalysed by basic materials, such as magnesium oxide, which are commonly included in commercial formulations.
Elimination takes place rapidly and probably occurs during milling when a
diamine curing system is used. Evidence for the presence of unsaturation in
amine-treated vinylidene fluoride-hexafluoropropylene copolymer comes
from the infrared spectrum of the material.
The second step in the vulcanization process is regarded as involving
addition of the curing agent at the sites of unsaturation. With a diamine
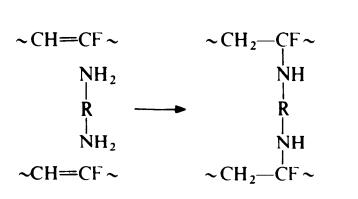
This reaction occurs during the press cure operation.
The third step in the vulcanization process is considered to involve the
elimination of hydrogen fluoride to form a diimine:
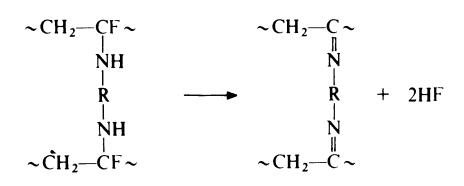
If this is the case, it would be necessary to remove all the water present in
order to force the equilibrium completely toward diimine formation and the
establishment of permanent cross-links. Experimental evidence for this pro�position is that a full state of cure is not attained if the long heating process is
carried out in the mould, i.e. in a virtually closed system. Hydrolysis is also
demonstrated by the fact that much of the diamine present in a vulcanizate
can be extracted by treatment with water. It may be noted here that
vinylidene fluoride-hexafluoropropylene copolymers which are peroxidecurable do not lead to water formation and so give vulcanizates with reduced
porosity.
Vulcanized vinylidene fluoride-hexafluoropropylene copolymers show excellent resistance to oils and fuels, both at room temperature and at elevated
temperature. Vulcanizates also have good resistance to most solvents, although polar solvents such as esters and ketones cause high swelling.
Vinylidene fluoride-hexafluoropropylene copolymer vulcanizates have excellent thermal stability, withstanding long periods at 250??C without serious
deterioration. Low temperature performance is limited and the elastomers
are not generally suitable for sub-zero use. Applications of the elastomers
include seals and hose in contact with fuels and lubricants, pump components
and tank linings.
A terpolymer of vinylidene fluoride, hexafluoropropylene and tetrafluoroethylene is also available and gives vulcanizates with improved re�sistance to heat ageing. | | Production Methods | Vinylidene fluoride-based elastomers have generally been
prepared by free-radical emulsion polymerization of the
monomers with organic or inorganic peroxide initiators.
Low-molecular-weight fluid or semifluid polymers have
been made with various chain transfer agents, special initiators,
or dehydrofluorination oxidation methods. | | Toxics Screening Level | The screening level for PVDF is 0.1 μg/m3 based on annual averaging. |
| | POLY(VINYLIDENE FLUORIDE-CO-HEXAFLUOROPROPYLENE) Preparation Products And Raw materials |
|