|
ChemicalBook Optimization Suppliers |
| 名前: |
shandongxinheng Gold |
| 電話番号: |
1526-9161022 15269161022 |
| 電子メール: |
15269161022@163.com |
|
| | ビスフェノールA Usage And Synthesis |
| 外観 | 白色の結晶性粉末 | | 溶解性 | 水に難溶。エタノール, アセトン, エーテルに易溶。エタノールに溶ける。 | | 解説 | ビスフェノールA,針状晶.融点156 ℃.かすかにフェノール臭を示し,刺激性がある.水に不溶,エタノール,アセトンに可溶.エポキシ樹脂,ポリ炭酸エステル樹脂,染料の合成原料に用いられる.
森北出版「化学辞典(第2版) | | 用途 | 可塑性ポリエステル原料,ポリカーボネート樹脂?エポキシ樹脂合成原料,塩化ビニル樹脂添加剤,ポリエステル樹脂中間体,難燃剤?熱硬化剤樹脂?塩ビ樹脂添加剤,インキ樹脂用?塗料?接着剤用?窯業鋳型用バインダー添加剤 (NITE CHRIP) | | 用途 | 合成中間体:
エポキシ樹脂の製造
TBBPAの製造
ポリカーボネートの製造消費者用及び産業用使用:
エポキシ樹脂の硬化剤
感熱紙中での使用
PVCから作られるアーティクルへの使用産業用の使用:
PVC加工のための抗酸化剤としての産業用使用 | | 用途 | 環境試料中の内分泌攪乱物質の分析(HPLC)用標準品、GC-MS分析標準品。 | | 用途 | ビスフェノールAは、フェノールとアセトンとの反応で製造され、エポキシ樹脂等のプラスチック原料や樹脂添加剤として用いられる。
合成樹脂の安定剤、酸化防止剤等として使用されます。
| | 製造 | アセトンとフェノールとを硫酸または濃塩酸の存在下に縮合させると得られるビスフェノールA. | | 説明 | Reports of bisphenol-
A sensitization, particularly in workers at epoxy
resin plants, are controversial. Bisphenol-A was also
reported as an allergen in fiberglass, semisynthetic
waxes, footwear and dental materials. | | 化学的特性 | Bisphenol A is a white or tan crystals or flakes with a mild phenolic odor and a very low vapor pressure (ECB, 2003). It is mildly soluble in water. It is not considered to be an explosive in the conventional sense but can pose a hazard as a finely powdered material in air (ECB, 2003). It is not considered to be a chemical oxidizer. | | 来歴 | Bisphenol A (BPA) was first synthesized in 1891, but it was not used widely until applications in the plastics industry were identified in the 1950s (University of Minnesota, 2008). While the most prominent use of BPA is in the manufacture of polycarbonate plastic and epoxy resins, it is also used in the production and processing of polyvinyl chloride (PVC) and modified polyamide and in the manufacture of carbonless and thermal paper, wood filler, adhesives, printing inks, surface coatings, polyurethane, brake fluid, resin-based dental composites and sealants, flame retardants, paints, and tires (ECB, 2003; EFSA, 2006).
 | | 使用 | A monomer used for policarbonate and epoxy resins; exhibits estrogenic activity. BPA is also used as a building block in polycarbonate bottles and in the epoxy-resin liners of metal cans. | | 使用 | Bisphenol A is used with epichlorhydrin for the
synthesis of epoxy resins bisphenol-A type. It leads
to bisphenol-A diglycidyl ether, which is the monomer
of bisphenol-A based epoxy resins. | | 使用 | Bisphenol A (BPA) is used as the constitutional monomer or the monomeric building block of polycarbonate plastics, either by trans-esterification with diphenyl carbonate or via the interfacial process with a monohydroxylic phenol. Together with epichlorohydrin, BPA is also used as a major component of epoxy resins. Bisphenol A-polycarbonate plastics are in turn used in the manufacture of plastic food containers such as reusable water bottles, while epoxy resins are used as inner linings of tin cans. In addition, BPA is also used as an additive in other plastics and polymers, particularly as an antioxidant or stabilizer in polyvinyl chloride, printer ink, and in some other products. | | 製造方法 | The formation of bisphenol A is thought to proceed as follows:
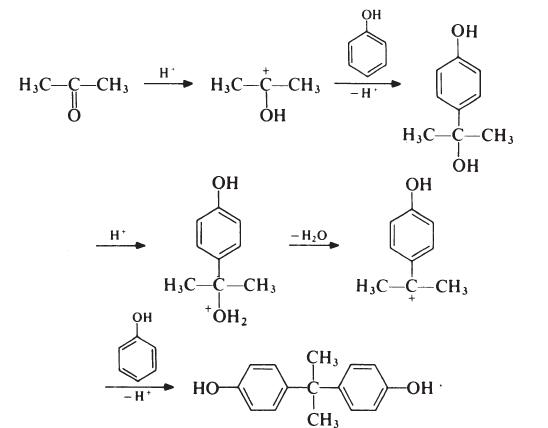
Although the reaction theoretically requires the molar ratio of reactants to be
2: 1, an improved yield of bisphenol A is obtained if additional phenol is
present; the optimum molar ratio is 4: 1. In a typical process, the phenol and
acetone are mixed and warmed to 50??C. Hydrogen chloride (catalyst) is
passed into the mixture for about 8 hours, during which period the temperature
is kept below 70??C to suppress the formation of isomeric products.
Bisphenol A precipitates and is filtered off and washed with toluene to remove
unreacted phenol (which is recovered). The product is then recrystallized from
aqueous ethanol. Since epoxy resins are oflow molecular weight and because
colour is not normally particularly important, the purity of bisphenol A used
in resin production is not critical. Material with a p,p'-isomer content of
95-98% is usually satisfactory; the principal impurities in such material are
o,p'- and o,o'-isomers. | | 定義 | ChEBI: A bisphenol that is 4,4'-methanediyldiphenol in which the methylene hydrogens are replaced by two methyl groups. | | Synthesis Reference(s) | Journal of the American Chemical Society, 71, p. 2287, 1949 DOI: 10.1021/ja01175a004 | | 一般的な説明 | White to light brown flakes or powder. Has a weak medicine odor. Sinks in water. | | 空気と水の反応 | The finely powdered resin is a significant dust explosion hazard. Insoluble in water. | | 反応プロフィール | Bisphenol A is incompatible with strong oxidizers. Bisphenol A is also incompatible with strong bases, acid chlorides and acid anhydrides. | | 危険性 | Poison; moderately toxic; teratogen;
irritant. | | 健康ハザード | Dusts irritating to upper respiratory passages; may cause sneezing. | | 火災危険 | Bisphenol A is combustible. Bisphenol A may form explosive dust clouds. Static electricity can cause its dust to explode. | | 燃焼性と爆発性 | Not classified | | 化学性质 | メタノールあるいは塩素系溶剤中で反応 | | 接触アレルゲン | Bisphenol A is used with epichlorhydrin for the
synthesis of epoxy resins bisphenol-A type, for
unsaturated polyester and polycarbonate resins, and
epoxy di(meth)acrylates. In epoxy resins, it leads to
bisphenol-A diglycidyl ether, which is the monomer
of bisphenol-A-based epoxy resins. Reports of
bisphenol-A sensitization are rare and concern
workers at epoxy resin plants, after contact with
fiber glass, semi-synthetic waxes, footwear, and
dental materials. It is also a possible sensitizer in
vinyl gloves. | | 職業ばく露 | Workers engaged in the manufacture
of epoxy, polysulfone, polycarbonate and certain polyester
resins. It is also used in flame retardants, rubber chemicals,
and as a fungicide. Bisphenol A (BP A), an environmental
estrogen, is found in a wide variety of products, including
polycarbonate bottles food and drink containers. According
to 2008 research conducted at University of Cincinnati,
when it comes to BPA, it’s not whether polycarbonate
bottles are new or old but the liquid’s temperature that
has the greatest impact on how much BPA is released.
When exposed to boiling hot water, BPA was released
55 times more rapidly than exposure to cold water. | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. | | 環境運命予測 | Bisphenol A can be released into the environment during the production, processing, and use of BPA-containing materials, although levels in environmental samples are generally very low or undetectable (ECB, 2003). This is because BPA has low volatility and a short half-life in the atmosphere, is rapidly biodegraded in water, and is not expected to be stable, mobile, or bioavailable from soils (ECB, 2003; Cousins et al., 2002).
Most environmental releases of BPA are during the manufacture of BPA-containing products when residual BPA in wastewater is released from treatment plants into receiving streams (Cousins et al., 2002). BPA's half-life in soil and water is in the order of 4.5 days while in air it is <1 day (Cousins et al., 2002). It has a low bioconcentration factor and is rapidly metabolized in fish, with a half-life of <1 day (Cousins et al., 2002). | | 貯蔵 | Color Code—Green: General storage may be used.Store away from heat and strong oxidizers and the incompatible materials listed above. | | 輸送方法 | UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous
hazardous material, Technical Name Required. | | 合成方法 | ビスフェノールAと臭素をメタノールあるいは塩素系溶剤中で反応させる | | 純化方法 | Crystallise bisphenol from acetic acid/water (1:1). It is used for making polycarbonate bottles and leaches out slowly on heating. It is a known “estrogenic chemical” shown to disrupt chemical signaling in the complex network of glands, hormones and cell receptors which make up the endocrine system. It causes low sperm count and damages the ecosystem by the feminisation of fish, reptiles and birds. [cf Chapter 1, p 3, Beilstein 6 IV 6717.] | | Toxicity evaluation | Bisphenol-A is a chemical substance with known oestrogenic action that is used in the manufacture of a wide range of products. The low-dose in utero exposure to bisphenol-A of experimental animals caused striking morphological changes in the vagina of postpubertal offspring. In addition, the oestrogen receptor alpha was not expressed during oestrus in the vagina of female offspring exposed to bisphenol-A and the altered vaginal morphology is attributed to the down regulation of oestrogen receptor alpha (Schonfelder et at., 2002). Another experiment on mice after intrauterine exposure to bisphenol-A showed differences in the rate of ductal migration into the stroma at 1 month of age and a significant increase in the percentage of ducts, terminal ducts, terminal end buds, and alveolar buds at 6 months of age. The changes in histoarchitecture, coupled with an increased presence of secretory product within alveoli, resemble those of early pregnancy. This suggests a disruption of the hypothalamic-pituitary-ovarian axis and/or mis-expression of developmental genes. It was concluded that the altered relationship in DNA synthesis between the epithelium and stroma and the increase in terminal ducts and terminal end buds are noteworthy, because these changes are associated with carcinogenesis in both rodents and humans (Markey et at., 2001). | | 不和合性 | Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, acid chlorides and acid
anhydrides. |
|