|
|
| | 1H-indole-3-propylamine Basic information | | Synthesis |
| Product Name: | 1H-indole-3-propylamine | | Synonyms: | 1H-indole-3-propylamine;1H-Indole-3-propan-1-amine;(1H-Indol-3-yl)-1-propanamine;Einecs 228-361-1;N-propyl-1H-indol-3-amine;1H-Indole-3-propanamine | | CAS: | 6245-89-2 | | MF: | C11H14N2 | | MW: | 174.24 | | EINECS: | 228-361-1 | | Product Categories: | | | Mol File: | 6245-89-2.mol | 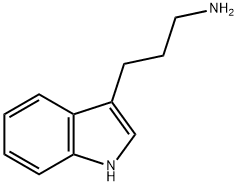 |
| | 1H-indole-3-propylamine Chemical Properties |
| Melting point | 64 °C | | Boiling point | 353.7±25.0 °C(Predicted) | | density | 1.125±0.06 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | pka | 17.29±0.30(Predicted) |
| | 1H-indole-3-propylamine Usage And Synthesis |
| Synthesis | A solution of LAH (1.0 M in ether, 36.5 mL, 36.5 mmol) was cooled to 0 °C, and a solution of the cyanoethyl indole (2.78 g, 16.3 mmol) was added slowly. Then, the solution was heated at reflflux for 3 h. It was cooled to 0 °C and quenched by dropwise addition of water (20 mL) followed by 1 N sodium hydroxide (40 mL). The phases were separated, and the aqueous phase was extracted with ether. The combined organic phases were washed with brine and then dried (potassium hydroxide). Evaporation of the solvent gave 2.6 g (92%) of homotryptamine as a yellow oil, which solidifified on standing. The hydrochloride was prepared by dissolving the amine in a minimum of ethanol and then a saturated solution of hydrogen chloride in ether was added until no additional salt formation was observed. The hydrochloride was recrystallized from ethanol.
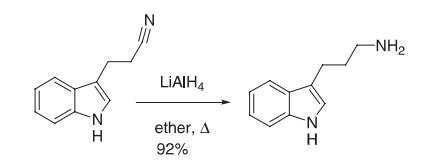
Reference: Kuehne, M. E.; Cowen, S. D.; Xu, F.; Borman, L. S. J. Org. Chem. 2001, 66, 5303–5316. | | Chemical Properties | Light yellow powder |
| | 1H-indole-3-propylamine Preparation Products And Raw materials |
|