|
|
| | (13α,17α)-7α-Acetoxy-21,23-epoxy-4,4,8-trimethyl-24-nor-5α-chola-1,14,20,22-tetren-3-one Basic information |
| Product Name: | (13α,17α)-7α-Acetoxy-21,23-epoxy-4,4,8-trimethyl-24-nor-5α-chola-1,14,20,22-tetren-3-one | | Synonyms: | (13α,17α)-7α-Acetoxy-21,23-epoxy-4,4,8-trimethyl-24-nor-5α-chola-1,14,20,22-tetren-3-one;Azadirone;24-Norchola-1,14,20,22-tetraen-3-one, 7-(acetyloxy)-21,23-epoxy-4,4,8-trimethyl-, (5α,7α,13α,17α)- | | CAS: | 25279-67-8 | | MF: | C28H36O4 | | MW: | 436.59 | | EINECS: | | | Product Categories: | | | Mol File: | 25279-67-8.mol | 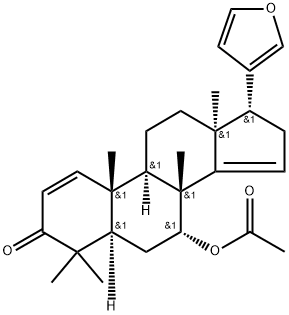 |
| | (13α,17α)-7α-Acetoxy-21,23-epoxy-4,4,8-trimethyl-24-nor-5α-chola-1,14,20,22-tetren-3-one Chemical Properties |
| Melting point | 123-125 °C | | Boiling point | 506.5±50.0 °C(Predicted) | | density | 1.15±0.1 g/cm3(Predicted) |
| | (13α,17α)-7α-Acetoxy-21,23-epoxy-4,4,8-trimethyl-24-nor-5α-chola-1,14,20,22-tetren-3-one Usage And Synthesis |
| Definition | ChEBI: A tetracyclic triterpenoid that is 4,4,8-trimethylandrosta-1,14-diene substituted by an oxo group at position 3, an acetoxy group at position 7 and a furan-3-yl group at position 17. Isolated from Azadirachta indica, it exhibits antiplasmodial
and antineoplastic activities. |
| | (13α,17α)-7α-Acetoxy-21,23-epoxy-4,4,8-trimethyl-24-nor-5α-chola-1,14,20,22-tetren-3-one Preparation Products And Raw materials |
|