- Bosutinib hydrate
-

- $33.00 / 5mg
-
2024-11-19
- CAS:918639-08-4
- Min. Order:
- Purity: 99.87%
- Supply Ability: 10g
- Bosutinib Monohydrate
-

- $2.00 / 1kg
-
2019-07-06
- CAS:918639-08-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: ask
|
| | Bosutinib Monohydrate Basic information |
| Product Name: | Bosutinib Monohydrate | | Synonyms: | Bosutinib Monohydrate;bosutinib hydrate;Bosutinib MonohydrateQ: What is
Bosutinib Monohydrate Q: What is the CAS Number of
Bosutinib Monohydrate;4-((2,4-Dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methylpiperazin-1-yl)propoxy)quinoline-3-carbonitrile hydrate | | CAS: | 918639-08-4 | | MF: | C26H31Cl2N5O4 | | MW: | 548.47 | | EINECS: | | | Product Categories: | | | Mol File: | 918639-08-4.mol |  |
| | Bosutinib Monohydrate Chemical Properties |
| form | Solid | | color | White to off-white |
| | Bosutinib Monohydrate Usage And Synthesis |
| Uses | Bosutinib hydrate is an oraly activel Src/Abl tyrosine kinase inhibito with IC50 of 1.2 nM and 1 nM, respectively[1]. | | Definition | ChEBI: A hydrate that is the monohydrate form of anhydrous bosutinib. | | Clinical Use | Bosulif ® (Bosutinib hydrate), also known as (SKI-606), is a novel 4-phenylamino-3-quinolinecarbonitrile kinase inhibitor approved for treatment of adults with chronic, accelerated, or blast
phase Philadelphia chromosome-positive chronic myeloid leukemia (Ph+CML). Bosutinib is an
orally-dosed, dual Src/Abl kinase inhibitor which provides an alternative treatment to patients
exhibiting immunity to imatinib and other kinase inhibitors utilized for this treatment. In contrast to
competitor tyrosine inhibitors, bosutinib inhibits autophosphorylation of both Srs and Abl kinases,
leading to decreased cell growth and apoptosis. Bosutinib was originally developed by Wyeth and
continues to be marketed by Pfizer after the merger of Wyeth and Pfizer in 2009. | | Synthesis | Several synthetic routes to bosutinib have been reported, including synthetic work for scale up and
processing to obtain pure salt forms of bosutinib for pharmaceutical applications.56-59 The current
manufacturing route begins with reaction of 2-methoxy-5-nitrophenol (36) and 1-bromo-3-
chloropropane (37) to provide aryl chloroether 38 in 82% yield. Reaction of 38 with Nmethylpiperazine
(39) and NaI in refluxing DME provided the functionalized aryl-nitro-piperazine 40
(77% yield), which was converted directly to aniline 41 under hydrogenolysis conditions. Aniline 41
was then reacted with triethyl orthoformate and aryl cyanoamide 42, which was generated in one step
from 2,4-dichloro-5-methoxy-aniline (44), 1,3-diisopropylcabodiimide (DIC), and cyanoacetic acid (45)
under refluxing conditions, to yield advanced intermediate 43 (93% over 2 steps). Finally,
conversion of 43 to bosutinib was facilitated by a POCl3-promoted cyclization in the presence of
sulfolane. As shown in Scheme 8, employment of carefully optimized conditions for the isolation of
bosutinib hydrate (VII) provided material in 75-82% yields and >99% purity. 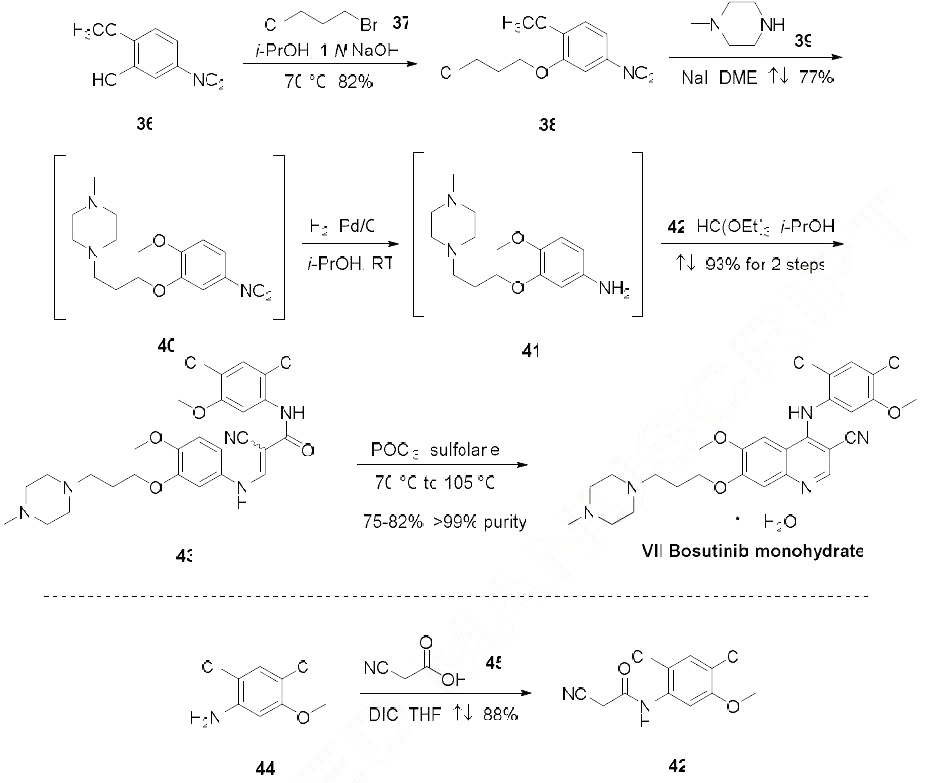
| | in vivo | Bosutinib (oral gavage; 75 mg/kg twice daily or 150 mg/kg once daily) hydrate has activity against human KU812 xenografts in nude mice. Bosutinib (150 mg/kg; once daily, 5 days weekly) hydrate has activity against syngeneic Bcr-Abl WT and mutant Ba/F3 xenografts[2]. | Animal Model: | KU812CM L xenograft model[2] | | Dosage: | 75 mg/kg twice daily or 150 mg/kg once daily | | Administration: | Bosutinib (oral gavage; 75 mg/kg twice daily or 150 mg/kg once daily) | | Result: | Had the therapeutic activity and produced a dose- and schedule-dependent weight loss. |
| Animal Model: | Syngeneic Bcr-Abl WT and mutant Ba/F3 xenografts[2] | | Dosage: | 150 mg/kg | | Administration: | Bosutinib (150 mg/kg; once daily, 5 days weekly) | | Result: | Decreased the rate of tumor growth and prolonged event-free survival of mice. |
| | References | [1] Jorge E Cortes, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012 Oct 1;30(28):3486-92. DOI:10.1200/JCO.2011.38.7522
[2] Miriam Puttini, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006 Dec 1;66(23):11314-22. DOI:10.1158/0008-5472.CAN-06-1199 |
| | Bosutinib Monohydrate Preparation Products And Raw materials |
|