| Company Name: |
ChemeGen(Shanghai) Biotechnology Co.,Ltd.
|
| Tel: |
18818260767 |
| Email: |
sales@chemegen.com |
| Products Intro: |
Product Name:U-51605
CAS:64192-56-9
Purity:98% Package:10 mg;50 mg;100 mg;500 mg;1 g;5 g;10 g
|
|
| | azo analog I Basic information |
| Product Name: | azo analog I | | Synonyms: | azo analog I;SRIZDZJPKIYUPZ-ACGFIOGVSA-N;5-Heptenoic acid, 7-[(1R,4S,5R,6R)-6-(1E)-1-octen-1-yl-2,3-diazabicyclo[2.2.1]hept-2-en-5-yl]-, (5Z)-;U-51605, PGI2 and TXA2 synthase inhibitor | | CAS: | 64192-56-9 | | MF: | C20H32N2O2 | | MW: | 332.48 | | EINECS: | | | Product Categories: | | | Mol File: | 64192-56-9.mol | 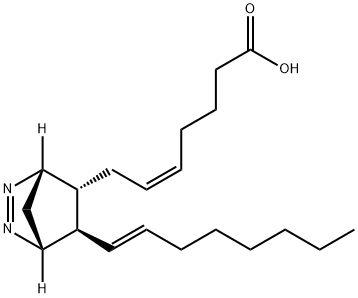 |
| | azo analog I Chemical Properties |
| Boiling point | 463.1±34.0 °C(Predicted) | | density | 1.10±0.1 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | DMF: >100 mg/ml (from U-46619); DMSO: >100 mg/ml (from U-46619); Ethanol: >100 mg/ml (from U-46619); PBS pH 7.2: >2 mg/ml (from U-46619) | | pka | 4.76±0.10(Predicted) |
| | azo analog I Usage And Synthesis |
| Uses | U-51605 is a platelet aggregation inhibitor and inhibits thromboxane synthesis. U-51605 is also a prostaglandin I2 synthase inhibitor and can inhibit the retinal vasodilation response induced by NO donors (such as NOR3)[1][2]. | | References | [1] Needleman P, Bryan B, Wyche A, et al. Thromboxane synthetase inhibitors as pharmacological tools: differential biochemical and biological effects on platelet suspensions[J]. Prostaglandins, 1977, 14(5): 897-907.
[2] Mori A, Namekawa R, Hasebe M, et al. Involvement of prostaglandin I2 in nitric oxide-induced vasodilation of retinal arterioles in rats[J]. European Journal of Pharmacology, 2015, 764: 249-255. |
| | azo analog I Preparation Products And Raw materials |
|