|
|
| | Vinflunine Ditartrate Basic information |
| Product Name: | Vinflunine Ditartrate | | Synonyms: | (2β,3β,4β,5α,12β,19α)-4-(Acetyloxy)-6,7-didehydro-15-[(2R,4R,6S,8S)-4-(1,1-difluoroethyl)-1,3,4,5,6,7,8,9-octahydro-8-(Methoxycarbonyl)-2,6-Methano-2H-azecino[4,3-b]indol-8-yl]-3-hydroxy-16-Methoxy-1-MethylaspidosperMidine-3-carboxylic Acid Methyl Ester (2R,3R)-2,3-Dihydroxybutanedioate;Ditartrate;20',20'-Difluoro-3',4'-dihydrovinorelbine Ditartrate;4'-Deoxy-20',20'-difluoro-5'-norvincaleukoblastine Ditartrate;BMS 710485;F 12158;Javlor;Vinflunine Ditartrate | | CAS: | 194468-36-5 | | MF: | C49H59F2N4O14 | | MW: | 966.0079664 | | EINECS: | | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 194468-36-5.mol |  |
| | Vinflunine Ditartrate Chemical Properties |
| Melting point | 244-246°C (dec) | | storage temp. | -20°C Freezer | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | White to Light Beige |
| | Vinflunine Ditartrate Usage And Synthesis |
| Chemical Properties | White Solid | | Uses | Vinflunine is a semisynthetic Vinca alkaloid with microtubule destabilizing and antiangiogenic activity. Vinflunine is a derivative of Vinorelbine. Vinflunine is used as an antineoplastic. | | Uses | Vinflunine Ditartrate can be used as Semisynthetic Vinca alkaloid with microtubule destabilizing and antiangiogenic activity; derivative of Vinorelbine. | | Clinical Use | Vinflunine ditartrate is a second generation difluorinated analog of the naturally-occuring substance
vinorelbine and it is approved for the treatment of non-small cell lung cancer, metastatic breast cancer
and ovarian cancer. Vinflunine, a tubulin polymerization inhibitor, belongs to the vinca alkaloid class
of anti-cancer agents. Introduction of the difluoro group of vinflunine dramatically improved antitumor
activity of the parent vinorelbine structure. Vinflunine was discovered by Pierre Fabre
Laboratories and in 2004 was licensed to Bristol-Myers Squibb for development and commercialization.
In 2007, the rights to venflunine were returned to Pierre Fabre which completed its development. | | Synthesis | Vinflunine can be prepared directly from vinorelbine (155) through the use of superacid chemistry. Reaction of 155 with antimony pentaflouride in hydrofluoric acid and Nbromosuccinimide
followed by treatment with two equivalents of tartaric acid produced vinflunine
ditartrate (XV) in 25% yield. An alternative synthesis of vinflunine was realized through reaction
of vinblastine or 3?ˉ,4?ˉ-dihydrovinblastine (156) with antimony pentaflouride and hydrofluoric acid in
chloroform to give the difluoro alkaloid 157 in 40% yield Ring contraction was effected by
reaction with trifluoroacetic acid and N-bromosuccinimide followed by aqueous sodium bicarbonate and
silver tetrafluoroborate to give vinflunine in 88% yield. Vinflunine ditartrate (XV) was prepared by
treating a solution of vinflunine in toluene with two equivalents of tartaric acid. 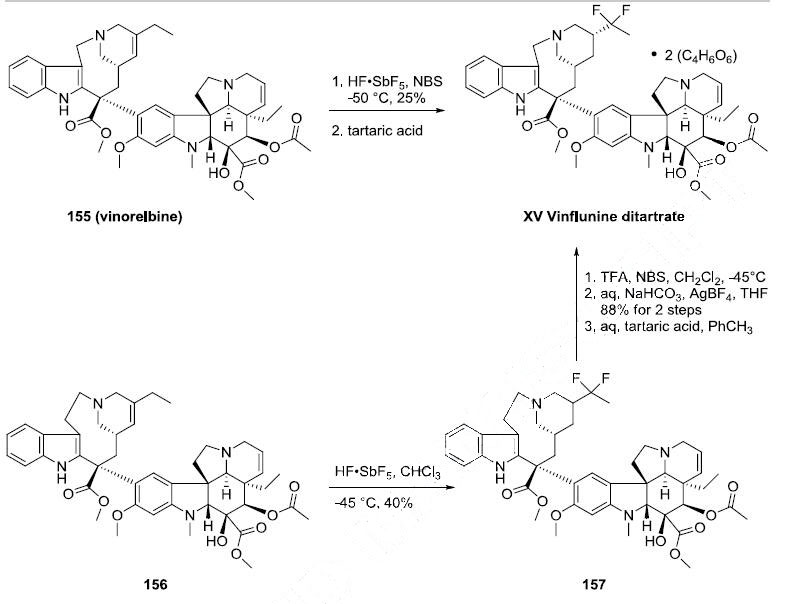
|
| | Vinflunine Ditartrate Preparation Products And Raw materials |
|