| Company Name: |
Suzhou Meishi Biotechnology Co., Ltd.
|
| Tel: |
1173954148q |
| Email: |
meishipharma@126.com |
| Products Intro: |
Product Name:431077-35-9
CAS:431077-35-9
Purity:99% HPLC Package:1G;5G;10G;100G;1KG;
|
| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
15002134094 |
| Email: |
marketing@targetmol.cn |
| Products Intro: |
Product Name:PPI-2458
CAS:431077-35-9
Purity:详情请点击官网 Package:1 mg
|
|
| | PPI-2458 Basic information |
| Product Name: | PPI-2458 | | Synonyms: | PPI 2458;PPI-2458;Carbamic acid, N-[(1R)-1-(aminocarbonyl)-2-methylpropyl]-, (3R,4S,5S,6R)-5-methoxy-4-[(2R,3R)-2-methyl-3-(3-methyl-2-buten-1-yl)-2-oxiranyl]-1-oxaspiro[2.5]oct-6-yl ester;QBDVVYNLLXGUGN-XGTBZJOHSA-N | | CAS: | 431077-35-9 | | MF: | C22H36N2O6 | | MW: | 424.53 | | EINECS: | | | Product Categories: | | | Mol File: | 431077-35-9.mol | 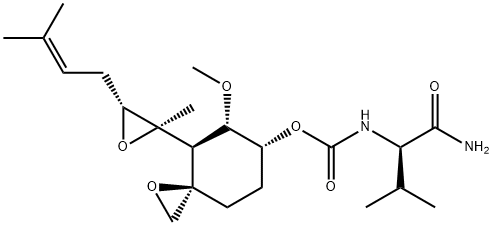 |
| | PPI-2458 Chemical Properties |
| Boiling point | 578.9±50.0 °C(Predicted) | | density | 1.17±0.1 g/cm3(Predicted) | | pka | 10.98±0.46(Predicted) |
| | PPI-2458 Usage And Synthesis |
| Enzyme inhibitor | This orally bioavailable, selectively cytotoxic agent (FWfree-acid = 424.54 g/mol; Photosensitive; Store in dark: IUPAC: [(3R,4S,5S,6R)-5-methoxy-4- [(2R,3R)-2-methyl-3-(3-methylbut-2-enyl)oxiran-2-yl]-1-oxaspiro2.5]octan- 6-yl] N-[(2R)-1-amino-3-methyl-1-oxobutan-2-yl]carbamate), a synthetic analogue of an Aspergillus fumigatus secondary metabolite (See Fumagillin), suppresses the formation of new blood vessels and inhibits endothelial cell proliferation and angiogenesis. Its epoxide group reacts with an active-site histidyl residue in methionyl aminopeptidase type II. Because removal of the N-terminal methionine from many proteins is required for their biologic activity, subcellular localization, and stability, methionyl-aminopeptidase plays a pivotal co-regulatory role in translation. PPI-2458 potently inhibits the proliferation of human fibroblast-like synoviocytes, or HFLS-RA (GI50 = 0.04 nM) derived from RA patients, showing >95% inhibition at 1 nM. Proliferation of human umbilical vein endothelial cells (HUVEC) is similarly inhibited (GI50 = 0.2 nM) by PPI-2458. Moreover, PPI-2458 inhibition of MetAP-2 catalysis (IC50 = 0.2 nM) in HFLS-RA is directly correlated with cell growth inhibition and a decrease in the DNA polymerase processivity factor PCNA. PPP-2458 also protects the a-subunit of eukaryotic initiation factor 2 from inhibitory phosphorylation. Based on the structure of TNP-470, a fumagillin analogue that exhibits dose-limiting CNS toxicity, PPI-2458 was designed to retain antiproliferative activity while improving its CNS toxicity profile. PPI-2458 also inhibits proliferation of B16F10 melanoma cells in vitro, (GI50 = 0.2 nM). This property, coupled with the absence of detectable resistance to PPI-2458 and the induction of morphological features of differentiated melanocytes, commends this agent for melanoma chemotherapy. PPI-2458 also inhibits non-Hodgkin's lymphoma cell proliferation in vitro and in vivo. Metabolic data demonstrate the participation of active metabolites in the in vivo efficacy of PPI-2458. |
| | PPI-2458 Preparation Products And Raw materials |
|