Odevixibat manufacturers
- Odevixibat
-

- $0.00 / 1g
-
2025-04-21
- CAS:501692-44-0
- Min. Order: 1g
- Purity: 98
- Supply Ability: 1000
|
| | Odevixibat Basic information |
| Product Name: | Odevixibat | | Synonyms: | A4250
(A-4250;Odevixibat);Butanoic acid, 2-[[(2R)-2-[[2-[[3,3-dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,2,5-benzothiadiazepin-8-yl]oxy]acetyl]amino]-2-(4-hydroxyphenyl)acetyl]amino]-, (2S)-;AZD 8294;Odevixibat(A4250);Unii-2W150K0uuc;AR-H 064974;AR-H064974 | | CAS: | 501692-44-0 | | MF: | C37H48N4O8S2 | | MW: | 740.93 | | EINECS: | | | Product Categories: | | | Mol File: | 501692-44-0.mol | 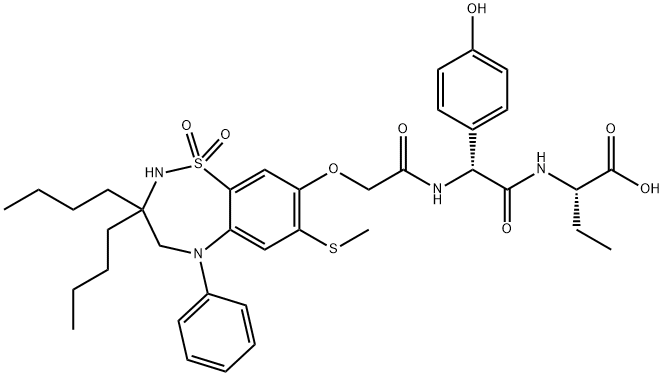 |
| | Odevixibat Chemical Properties |
| density | 1.34±0.1 g/cm3(Predicted) | | storage temp. | Store at -20°C, stored under nitrogen | | solubility | DMSO : 166.67 mg/mL (224.95 mM; Need ultrasonic) | | form | A solid | | pka | 3.32±0.10(Predicted) | | color | White to off-white |
| | Odevixibat Usage And Synthesis |
| Uses | Odevixibat (A4250) is a selective and orally active ileal apical sodium-dependent bile acid transporter (ASBT) inhibitor. Odevixibat decreases cholestatic liver and bile duct injury in mice model. Odevixibat has the potential for the treatment of primary biliary cirrhosis[1]. | | Biological Activity | Odevixib at is an orally active, potent and selective ileal bile acid transporter (IBAT; ASBT; SLC10A2) inhibitor th at improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis (0.01% w/w in diet). | | in vivo | Odevixibat (A4250)(0.01% (w/w) in chow diet; 4 weeks) improves sclerosing cholangitis and significantly reduces serum alanine aminotransferase, alkaline phosphatase and BAs levels, hepatic expression of pro-inflammatory and pro-fibrogenic genes and bile duct proliferation in Mdr2-/- mice[1].
In addition, Odevixibat (A4250) significantly reduces bile flow and biliary BA output, which correlates with reduced bsep transcription, while Ntcp and Cyp7a1 are induced[1]. | Animal Model: | Eight week old Mdr2-/- (Abcb4-/-) mice (model of cholestatic liver injury and sclerosing cholangitis)[1] | | Dosage: | 0.01% (w/w) in chow diet | | Administration: | 4 weeks | | Result: | Decreased cholestatic liver and bile duct injury in mice model. |
| | References | [1] Baghdasaryan A, et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis.J Hepatol. 2016 Mar;64(3):674-81. DOI:10.1016/j.jhep.2015.10.024 |
| | Odevixibat Preparation Products And Raw materials |
|