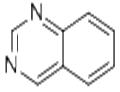
- Quinazoline
-
- $98.00 / 1KG
-
2019-07-06
- CAS:253-82-7
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 100KG
|
| | Quinazoline Basic information |
| Product Name: | Quinazoline | | Synonyms: | 1,3-Diazanaphthalene;4.5-Benzopyrimidine;Benzo(e)pyrimidine;1,3-Benzodiazine, Benzopyrimidine;Quinazoline ,98%;Benzo[d]pyrimidine;Benzopyrimidine;Quinazoline (6CI,8CI,9CI) | | CAS: | 253-82-7 | | MF: | C8H6N2 | | MW: | 130.15 | | EINECS: | 205-965-3 | | Product Categories: | PYRIMIDINE | | Mol File: | 253-82-7.mol | 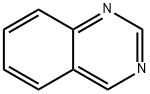 |
| | Quinazoline Chemical Properties |
| Melting point | 46-48 °C (lit.) | | Boiling point | 243 °C (lit.) | | density | 1.2002 (rough estimate) | | refractive index | 1.6231 (estimate) | | Fp | 224 °F | | storage temp. | Sealed in dry,Room Temperature | | solubility | H2O: freely soluble | | pka | 3.43(at 20℃) | | form | Crystalline Powder, Crystals and/or Chunks | | color | Yellow to beige to brownish | | Water Solubility | H2O: freely soluble
organic solvents: soluble | | Merck | 14,8053 | | BRN | 109370 | | CAS DataBase Reference | 253-82-7(CAS DataBase Reference) | | NIST Chemistry Reference | Quinazoline(253-82-7) | | EPA Substance Registry System | Quinazoline (253-82-7) |
| | Quinazoline Usage And Synthesis |
| Chemical Properties | Beige to brownish crystalline powder | | Uses | Quinazoline was used to study the electrochemical behaviour of quinazoline using modern polarographic and voltammetric methods. It is the basic structural unit of pharmaceuticals and plays an important role in modern synthesis of antitumor drugs. | | Definition | ChEBI: A mancude organic heterobicyclic parent that is naphthalene in which the carbon atoms at positions 1 and 3 have been replaced by nitrogen atoms. | | General Description | Quinazolines has applications in medicinal chemistry due to their antibacterial, antifungal, anticonvulsant, anti-inflammatory and antitumor activities. It is the basic structural unit of pharmaceuticals and plays an important role in modern synthesis of antitumor drugs. | | Biochem/physiol Actions | Genotoxicity of quinazoline was established by bacterial SOS Chromotest (Escherichia Coli). | | Purification Methods | Purify quinazoline by passage through an activated alumina column in *C6H6 or pet ether (b 40-60o). Distil it under reduced pressure, sublime it under vacuum and crystallise it from pet ether. The picrate has m 188-189o (from MeOH). [Armarego J Appl Chem 11 70 1961, Armarego Quinazolines, Fused Pyrimidines Part I Brown Ed, Wiley-Interscience 1967, Brown Quinazolines Supplement I Taylor Ed, Wiley-Interscience 1996, ISBN 0-471-14565-3; for covalent hydration see Albert & Armarego Adv Heterocycl Chem 4 1 1965, Beilstein 23 H 175, 23 II 177, 23 III/IV 1221.] |
| | Quinazoline Preparation Products And Raw materials |
| Raw materials | 5-Nitroisoquinoline-->Quinazoline, 1,4-dihydro--->4-Quinazolinecarboxamide-->4-Quinazolinemethanol, α-(4-methoxyphenyl)-α-methyl--->ORTHO-NITROBENZALDEHYDEDIMETHYLACETAL-->2,3,4,5-TETRAHYDRO-1H-BENZO[E][1,4]DIAZEPINE-->2(1H)-Quinazolinone (6CI,8CI,9CI)-->2,1-BENZISOXAZOLE-->2-AMINOBENZYLAMINE | | Preparation Products | 7-chloro-1,3-dihydro-5-phenyl-2-oxo-2H-1,4-benzodiazepin-3-yl acetate-->Demoxepam-->4-Chloroquinazoline-->Bunazosin hydrochloride-->Quinapril-->Terazosin-->Doxazosin-->Alfuzosin hydrochloride-->QUINAZOLIN-4-YLAMINE |
|