| Company Name: |
SynthoCascade Research Laboratories
|
| Tel: |
+91-9440962500 +91-9440962500 |
| Email: |
synthocascade@gmail.com |
| Products Intro: |
Product Name:4-hydroxy-3-iodo acetophenone
CAS:62615-24-1
Purity:98% Package:1 kg,5 kg, 10 kg,25kg and 1 MT
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Email: |
orderCN@merckgroup.com |
| Products Intro: |
Product Name:4'-Hydroxy-3'-iodoacetophenone
CAS:62615-24-1
Purity:97% Package:5G Remarks:722057-5G
|
|
| | 2-Iodo-4-acetylphenol Basic information |
| Product Name: | 2-Iodo-4-acetylphenol | | Synonyms: | 2-Iodo-4-acetylphenol;3'-Iodo-4'-hydroxyacetophenone;4'-Hydroxy-3'-iodoacetophenone;4'-Hydroxy-3'-iodoacetophenone 97%;4-Acetyl-2-iodophenol;Ethanone, 1-(4-hydroxy-3-iodophenyl)-;1-(4-Hydroxy-3-iodo-phenyl)-ethanone;1-(4-hydroxy-3-iodophenyl)ethan-1-one | | CAS: | 62615-24-1 | | MF: | C8H7IO2 | | MW: | 262.04 | | EINECS: | | | Product Categories: | | | Mol File: | 62615-24-1.mol | 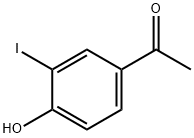 |
| | 2-Iodo-4-acetylphenol Chemical Properties |
| Melting point | 155-160°C | | form | powder |
| Hazard Codes | Xn | | Risk Statements | 22-41 | | Safety Statements | 26-39 | | WGK Germany | 1 |
| | 2-Iodo-4-acetylphenol Usage And Synthesis |
| Uses | Reactant for:
- Preparation of cyclopentene fused benzofurans and indoles via Pd-catalyzed tandem ring opening-ring closing reaction with diazabicyclic alkenes
- Highly regioselective synthesis of spirocyclic compounds by a palladium-catalyzed intermolecular tandem reaction
- Regioselective synthesis of indene derivatives via Pd/C-catalyzed cyclization reaction in air
- Preparation of carbazoles via cross-coupling with silylaryl triflates followed by palladium-catalyzed cyclization
- Stereoselective preparation of heteropolycyclic compounds via palladium-catalyzed stereoselective heteroannulation of with cyclic alkenes
- Preparation of disubstituted coumarins via palladium-catalyzed carbonylative annulation of internal alkynes
- Preparation of arylalkynes, benzofurans and indoles via Sonogashira coupling and cyclization on alumina
| | Preparation | Preparation by reaction of iodine and potassium iodide on 4-hydroxyacetophenone in aqueous ammonium hydroxide at r.t. (54–57%). | | Synthesis Reference(s) | Journal of Medicinal Chemistry, 23, p. 738, 1980 DOI: 10.1021/jm00181a008 |
| | 2-Iodo-4-acetylphenol Preparation Products And Raw materials |
|