|
|
| | (1S-cis)-Milnacipran Hydrochloride Basic information |
| | (1S-cis)-Milnacipran Hydrochloride Chemical Properties |
| storage temp. | Store at -20°C | | solubility | DMSO:57.0(Max Conc. mg/mL);201.55(Max Conc. mM) | | form | A crystalline solid | | color | White to off-white |
| | (1S-cis)-Milnacipran Hydrochloride Usage And Synthesis |
| Description | Levomilnacipran (Fetzima®) is a dual serotonin–norepinephrine
reuptake inhibitor (SNRI) approved by the FDA in 2013 for the
treatment of major depressive disorders (MDD).
Levomilnacipran is the most active enantiomer of the racemate
Milnacipran, which is currently used to treat pain associated
with fibromyalgia. The drug was developed by Forest
Laboratories and the Pierre Fabre group. | | Uses | The (1S-cis)-enatiomer of Milnacipran (M344600). A selective norepinephrine and serotonin reuptake inhibitor approved for the management of fibromyalgia. | | Uses | The S-enatiomer of Milnacipran (M344600). | | Synthesis | Reaction of phenylacetonitrile (100) and commercially available
(R)-epichlorohydrin (101) with NaNH2 led to chloride displacement
and intramolecular cyclopropanation, yielding lactone 102
after a one-pot nitrile hydrolysis and acid-promoted lactonization (75% yield over 3 steps). Lactone ring-opening with Et2NH¨CAlCl3
complex provided amido-alcohol 103, which was converted to its
phthalimido derivative 104 by sequential treatment with thionyl
chloride and potassium phthalimide in 80% over three steps.
Finally, levomilnacipran hydrochloride (XIII) was obtained in
>95% optical purity after phthalimide cleavage, HCl salt formation,
and crystallization from HCl/i-PrOH/i-PrAc. This sequence represents
a highly efficient route to levomilnacipran, requiring no isolation
of intermediates, resulting in >40% overall yield, and
allowing use of the same solvent solution (toluene) for all steps. 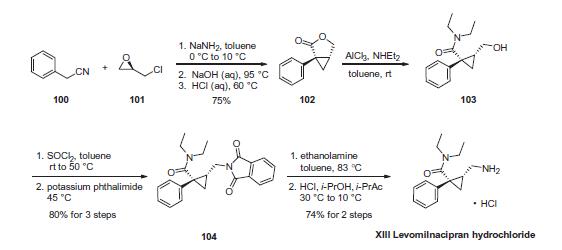
|
| | (1S-cis)-Milnacipran Hydrochloride Preparation Products And Raw materials |
|