oxyphencyclimine manufacturers
- Oxyphencyclimine
-
- $1520.00 / 25mg
-
2024-10-28
- CAS:125-53-1
- Min. Order:
- Purity:
- Supply Ability: 10g
- oxyphencyclimine
-

- $0.00 / 1kg
-
2023-09-14
- CAS:125-53-1
- Min. Order: 1kg
- Purity: 99.87%
- Supply Ability: 20tons
|
| | oxyphencyclimine Basic information |
| Product Name: | oxyphencyclimine | | Synonyms: | Caridan;Naridan;Zamanil;C07851;1,4,5,6-Tetrahydro-1-methyl-2-pyrimidinemethanol alpha-phenylcyclohexaneglycolate (ester);Antulcus;Benzeneacetic acid, α-cyclohexyl-α-hydroxy-, (1,4,5,6-tetrahydro-1-methyl-2-pyrimidinyl)methyl ester;Manir | | CAS: | 125-53-1 | | MF: | C20H28N2O3 | | MW: | 344.45 | | EINECS: | 2047433 | | Product Categories: | | | Mol File: | 125-53-1.mol | 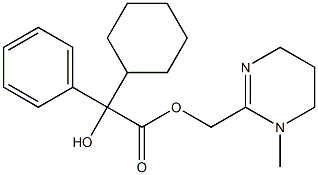 |
| | oxyphencyclimine Chemical Properties |
| | oxyphencyclimine Usage And Synthesis |
| Originator | Vio-Thene ,Rowell,US,1959 | | Uses | Antulcus can be used as a potential inhibitor for insulin fibril formation. | | Definition | ChEBI: Oxyphencyclimine is a monocarboxylic acid. | | Manufacturing Process | To a stirred solution of 8.8 grams (0.1 mol) of 1,3-diaminobutane in 150 ml of ethanol maintained at 0° to 5°C, there was added 25.8 grams (0.1 mol) of ethyl chlorimidoacetate hydrochloride during a period of 20 minutes. After the mixture had been stirred at 0° to 5°C for two hours, it was acidified at this temperature by the addition of ethanolic hydrogen chloride. The mixture was warmed to room temperature and filtered to remove 4.3 grams of solid ammonium chloride. The filtrate was concentrated to approximately 40 ml, filtered and refrigerated. The solid which separated was isolated, washed with acetone and dried. There was obtained 7.4 grams (40% of the theoretical yield) of 2-chloromethyl-4-methyl-1,4,5,6-tetrahydropyrimidine hydrochloride melting at 158° to 160°C.
In a second step, cyclohexyl bromide was reacted with magnesium, then with benzoyl formic acid to give cyclohexylphenyl glycolic acid. A solution of 1.8 grams (0.01 mol) of 2-chloromethyl-1-methyl-1,4,5,6-tetrahydropyrimidine hydrochloride in 5 ml of water was made alkaline with 5 ml of 50% NaOH and extracted with ether. The ether solution, which contained the basic chloride, was dried over calcium sulfate and added to a solution of 2.3 grams (0.01 mol) of α-cyclohexylphenylglycolic acid in 75 ml of isopropanol. The solution was distilled to remove the ether, and 0.1 gram of powdered potassium iodide added to the residual isopropanol solution which was then refluxed for 6 hours. The solid which had separated was redissolved by the addition of 20 ml of ethanol and the solution charcoaled, concentrated, and cooled. The solid which separated, 1-methyl-1,4,5,6-tetrahydro-2-pyrimidylmethyl αcyclohexylphenyl-glycolate hydrochloride, weighed 1.4 grams and melted at 228° to 229°C with decomposition after recrystallization from ethanol. | | Brand name | Daricon (Pfizer). | | Therapeutic Function | Spasmolytic |
| | oxyphencyclimine Preparation Products And Raw materials |
|