|
|
| | (20S)-16,17-Didehydro-9,17-dihydro-9,20-dihydroxy-12-methoxyibogamine-18-carboxylic acid methyl ester Basic information |
| Product Name: | (20S)-16,17-Didehydro-9,17-dihydro-9,20-dihydroxy-12-methoxyibogamine-18-carboxylic acid methyl ester | | Synonyms: | (20S)-16,17-Didehydro-9,17-dihydro-9,20-dihydroxy-12-methoxyibogamine-18-carboxylic acid methyl ester;Voacristine hydroxyindolenine;9,20-dihydroxy-12-methoxy-16,17-didehydro-9,17-dihydro-ibogamine-18-carboxylic acid methyl ester;Ibogamine-18-carboxylic acid, 16,17-didehydro-9,17-dihydro-9,20-dihydroxy-12-methoxy-, methyl ester, (9β,20S)- | | CAS: | 15215-86-8 | | MF: | C22H28N2O5 | | MW: | 400.47 | | EINECS: | | | Product Categories: | | | Mol File: | 15215-86-8.mol | 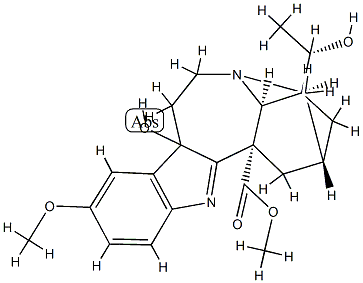 |
| | (20S)-16,17-Didehydro-9,17-dihydro-9,20-dihydroxy-12-methoxyibogamine-18-carboxylic acid methyl ester Chemical Properties |
| | (20S)-16,17-Didehydro-9,17-dihydro-9,20-dihydroxy-12-methoxyibogamine-18-carboxylic acid methyl ester Usage And Synthesis |
| Description | This alkaloid is produced by irradiating Voacristine but also occurs naturally in
the root bark of Ervatamia dichotoma, remaining in the mother liquors after
removal of the phenolic bases, from which it may be obtained by chromatographic
separation. The structure has been determined from the infrared and
NMR spectra. | | References | Schnoes et al., J. Org. Chem., 33, 1225 (1968) |
| | (20S)-16,17-Didehydro-9,17-dihydro-9,20-dihydroxy-12-methoxyibogamine-18-carboxylic acid methyl ester Preparation Products And Raw materials |
|