|
|
| | Diphenylvinylsulfonium Triflate Basic information |
| Product Name: | Diphenylvinylsulfonium Triflate | | Synonyms: | SulfoniuM, ethenyldiphenyl-, 1,1,1-trifluoroMethanesulfonate (1:1);ethenyldiphenylsulfanium trifluoromethanesulfonate;SulfoniuM, ethenyldiphenyl-, 1,1,1-trifluoroMethanesulfonate;ethenyl(diphenyl)sulfanium;Diphenyl(vinyl)sulfonium trifluoromethanesulfonate;Diphenylvinylsulfonium triflate;Sulfonium hanesulfonate (1:1) | | CAS: | 247129-88-0 | | MF: | C15H13F3O3S2 | | MW: | 362.38 | | EINECS: | | | Product Categories: | | | Mol File: | 247129-88-0.mol | 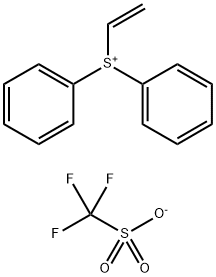 |
| | Diphenylvinylsulfonium Triflate Chemical Properties |
| storage temp. | Sealed in dry,Room Temperature |
| | Diphenylvinylsulfonium Triflate Usage And Synthesis |
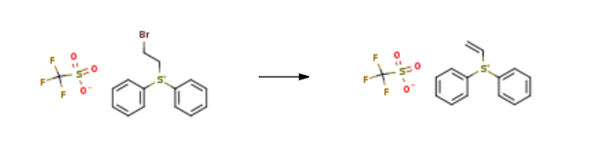
| Description | Diphenylvinylsulfonium Triflate is a pale yellow, oily organic compound. It has a vinylsulfonium salt structure, and nucleophiles readily undergo conjugate addition reactions with vinylsulfonium salts to form thionimide intermediates, which can undergo a series of further transformations. Applications in epoxidation, azide and other cyclisation reactions have demonstrated the broad applicability of vinyl sulfonium salts as two-carbon bridges. | | Uses | Diphenylvinylsulfonium Triflate can be used as GLP-??1r modulating compounds to treat GLP-??1R mediated diseases such as metabolic and related diseases, including but not limited to NASH, obesity, and type 2 diabetes. | | Synthesis | Diphenylvinylsulfonium Triflate is synthesised using (2-bromoethyl)diphenylsulfonium trifluoromethanesulfonate as a raw material by chemical reaction. The specific synthesis steps are as follows:
INTERMEDIATE 75(DiphenylXvmyDsulfonium trifluoromethanesulfonateIntermediate 74 (11.4 g, 25.7 mmol) was dissolved in THF/H2O (2:1) (42 mL). KHCO3 (3.1 g, 30.9 mmol) was added and the reaction mixture was stirred for 20 minutes at r.t. The solvent was evaporated immediately under reduced pressure (using a rotary evaporator connected to a high vacuum pump and keeping the water-bath temperature below 2O0C). The reaction mixture was then redissolved in DCM (40 mL), dried over MgSO4, filtered and evaporated. The residue was redissolved in DCM (10 mL) and loaded onto a silica bed (4 cm depth and 2.5 cm diameter). The resulting band was covered with 1 cm sand and eluted with DCM (400 mL), followed by 10% MeOH in DCM (200 mL). The product was isolated as a light brown oil (9.53 g, quantitative). 6H (CDCl3) 7.90-7.87 (4H, m), 7.79-7.66 (6H, m), 7.53 (IH, dd, J 8.8, 8.8 Hz), 6.69 (IH, dd, J 9.5, 2.5 Hz), 6.55 (IH, dd, J 16.5, 2.5 Hz).
 |
| | Diphenylvinylsulfonium Triflate Preparation Products And Raw materials |
|