| Company Name: |
MedChemexpress LLC
|
| Tel: |
021-58955995 |
| Email: |
sales@medchemexpress.cn |
| Products Intro: |
Product Name:SA504
CAS:35035-05-3
Purity:400RMB/1mg Package:>98%
|
| Company Name: |
Jinan Ruisi Pharmaceutical Technology Co., LTD
|
| Tel: |
0531-69900633 18340090523 |
| Email: |
changyuan20@163.com |
| Products Intro: |
Product Name:Piperidinium, 3-(di-2-thienylmethylene)-5-methoxy-1,1-dimethyl-, bromide (1:1)
CAS:35035-05-3
Purity:98% Package:500Mg;1g;5g;25g;100g,5KG;1KG
|
|
| | Timepidium bromide Basic information |
| Product Name: | Timepidium bromide | | Synonyms: | TIMEPIDIUM BROMIDE;TIMEPIDIUM;1,1-Dimethyl-5-methoxy-3-(dithien-2-ylmethylene)piperidinium bromide;Mepidium;Piperidinium, 3-(di-2-thienylmethylene)-5-methoxy-1,1-dimethyl-, bromide (9CI);SA 504;SA 50Y;Sesden | | CAS: | 35035-05-3 | | MF: | C17H22BrNOS2 | | MW: | 400.4 | | EINECS: | | | Product Categories: | | | Mol File: | 35035-05-3.mol | 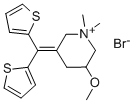 |
| | Timepidium bromide Chemical Properties |
| Melting point | 198-200° | | storage temp. | Store at -20°C | | solubility | Soluble in DMSO |
| | Timepidium bromide Usage And Synthesis |
| Originator | Sesden,TANABE SEIYAKU,Japan,1976 | | Definition | ChEBI: Timepidium bromide is an organic molecular entity. | | Manufacturing Process | 120 g of 5-hydroxynicotinic acid are dissolved in 1 liter of methanol. After
saturating with dry-hydrogen chloride gas at 0°C, the solution is refluxed for 2
hours. Then, the solution is concentrated to dryness. The residue thus
obtained is dissolved in water. The solution is neutralized with sodium
bicarbonate. The precipitated crystals are collected by filtration, washed with
water and then dried. 126 g of methyl 5-hydroxynicotinate are obtained.
Yield: 93%. Melting point 184°C to 186°C.
460 g of methyl 5-hydroxynicotinate and 621 g of potassium carbonate are
suspended in 200 ml of tetrahydrofuran-methanol (4:1). 1,134 g of dimethyl
sulfate are added dropwise to the suspension in nitrogen atmosphere at room
temperature. The mixture is stirred overnight at the same temperature and
then filtered. The filtrate is concentrated to dryness. The residue thus
obtained is mixed with 1.6 liters of methanol and 280 ml of Raney-nickel, and
hydrogenated overnight in an autoclave at room temperature and at a
pressure of 85 atmospheres. 200 g of Raney-nickel are added to the reaction
mixture. The mixture is adjusted to pH 9.5 with triethylamine, and is further
subjected to hydrogenation for 20 hours in an autoclave at 70°C and at a
pressure of 100 atmospheres. Potassium carbonate and a small amount of ice
are added to the reaction mixture to bring the pH to 11. The mixture is
extracted with ether. After drying, the ether layer is filtered. The filtrate is evaporated to remove ether. The residue thus obtained is distilled under
reduced pressure. 450 g of methyl N-methyl-5-methoxynipecotinate are
obtained. Yield: 80%. Boiling point 80°C to 81°C/0.5 mm Hg.
A solution of 18 g of 2-thienyl bromide in 30 ml of tetrahydrofuran is
gradually added to a mixture of 2.6 g of magnesium and 80 ml of
tetrahydrofuran under stirring at 50°C. The mixture is stirred for 5 hours at
room temperature until the magnesium is entirely dissolved in the solution.
6.2 g of methyl N-methyl-5-methoxy-nipecotinate are added to the mixture.
Then, the mixture is refluxed for 4 hours. After the reaction is completed,
tetrahydrofuran is distilled off under reduced pressure. An aqueous
ammonium chloride solution is added to the residue, and the solution is
extracted with chloroform. The extract is dried and then evaporated to remove
chloroform. The viscous oil thus obtained is recrystallized from a mixture of
benzene and ether. 7 g of di-(2-thienyl)-(N-methyl-5-methoxy-3-piperidyl)-
carbinol are obtained as crystals. Melting point 142°C to 146°C.
7 g of the product are dissolved in 150 ml of 10% hydrochloric acid, and the
solution is heated at 80°C for 30 minutes. After the reaction is completed, the
solution is basified with sodium hydroxide and then extracted with ether. The
extract is washed with water, dried and evaporated to remove ether. 5 g of di-
(2-thienyl)-(N-methyl-5-methoxy-3-piperidylidene)-methane are obtained as
pale yellow oil.
365 mg of di-(2-thienyl)-(N-methyl-5-methoxy-3-piperidylidene)-methane are
dissolved in 15 ml of ether. 1 ml of methyl bromide is added to the solution.
Then, the solution is stirred overnight. The precipitated crystals are collected
by filtration and recrystallized from a mixture of acetone and ether. 390 mg of
di-(2-thienyl)-(N-methyl-5-methoxy-3-piperidylidene)-methane methyl
bromide are obtained as colorless crystals. Melting point 198°C to 200°C. | | Therapeutic Function | Anticholinergic | | in vivo | Effects of Timepidium bromide (TB), acetylcholine (ACh) and neostigmine (Neost) on gastric and duodenal blood flow distribution are studied by the use of 131 I-labeled macroaggregated human serum albumin (MAA) in rabbits. In normal rabbits, gastric blood flow is found to be uneven in various regions of the stomach: anterior corpus (50% of total gastric blood flow) greater than posterior corpus (40%) greater than pyloric antrum (7%). Intravenous administration of Timepidium bromide (200 μg/kg) to normal rabbits produces a slight increase in total gastric blood flow, but the increase in the mucosal layer of the pyloric antrum is considerable. |
| | Timepidium bromide Preparation Products And Raw materials |
|