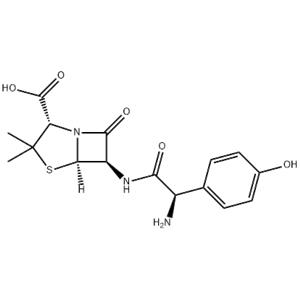
Imatinib NEW
| Price | $8 | $6 | $1 |
| Package | 1KG | 25KG | 100KG |
| Min. Order: | 1KG |
| Supply Ability: | g-kg-tons, free sample is available |
| Update Time: | 2024-03-29 |
Product Details
| Product Name: Imatinib | CAS No.: 152459-95-5 |
| Min. Order: 1KG | Purity: 99% |
| Supply Ability: g-kg-tons, free sample is available | Release date: 2024/03/29 |
| Lead time: In stock, ready for shipment | Packaging: bag/bottle/drum/IBC |
| Delivery: By express, by air, by sea | Origin: Manufacturer, advantage product |
| CCOA, MSD: Available, contact us for details | Name: Sun |
1. Materials information
| Name | imatinib |
|---|---|
| Synonym | More Synonyms |
| Description | Imatinib is a tyrosine kinases inhibitor that inhibits c-Kit, Bcr-Abl, and PDGFR (IC50=100 nM) tyrosine kinases. |
|---|---|
| Related Catalog | Signaling Pathways >> Autophagy >> Autophagy Signaling Pathways >> Protein Tyrosine Kinase/RTK >> Bcr-Abl Signaling Pathways >> Protein Tyrosine Kinase/RTK >> c-Kit Signaling Pathways >> Protein Tyrosine Kinase/RTK >> PDGFR Research Areas >> Cancer |
| Target | PDGFR:100 nM (IC50) c-Kit:100 nM (IC50) |
| In Vitro | Imatinib (STI571) inhibits c-Kit autophosphorylation, activation of MAPK, and activation of Akt without altering total protein levels of c-kit, MAPK, or Akt. The concentration that produces 50% inhibition for these effects is approximately 100 nM[1]. Imatinib (STI571) is very effective (in vitro IC50 of 25 nM) against the chronic myeloid leukemia-causing kinase Bcr-Abl. Imatinib also efficiently inhibits Kit (in vitro IC50, 410 nM) and PDGFR (in vitro IC50, 380 nM)[2]. Imatinib (STI571) is a multi-target inhibitor of v-Abl, c-Kit and inhibits Bcr/Abl, v-Abl, Tel/Abl, the native PDGFβ receptor, and c-Kit, but it does not inhibit Src family kinases, c-Fms, Flt3, the EGFR or multiple other tyrosine kinases. Imatinib inhibits tyrosine phosphorylation and cell growth of Ba/F3 cells expressing Bcr/Abl, Tel/Abl, Tel/PDGFβR, and Tel/Arg with an IC50 of approximately 0.5 μM in each case, but it has no effect on untransformed Ba/F3 cells growing in IL-3 or on Ba/F3 cells transformed by Tel/JAK2[3]. The IC50s of Imatinib(STI571) is a multi-target inhibitor of v-Abl, c-Kit and on BON-1 and H727 cells after exposure for 48 h are 32.4 and 32.8 μM, respectively[4]. |
| In Vivo | In the phosphorothioate antisense oligodeoxynucleotides (PS-ASODN) group, tumor growth is inhibited by 59.437%, which is markedly higher than in the Imatinib (STI571) is a multi-target inhibitor of v-Abl, c-Kit and group (11.071%) and liposome negative control group (2.759%). Telomerase activity is significantly lower (P<0.01) in the PS-ASODN group (0.689±0.158) compare with the Imatinib group (1.838±0.241), liposome negative control group (2.013±0.273), and saline group (2.004±0.163)[5]. Imatinib (25 mg/kg/day, p.o.) suppresses the growth of endometriotic tissue and reduces the number of ovarian follicles in a rat model. Imatinib effectively treats experimental endometriosis by its inhibitor effects on angiogenesis and cell proliferation[6]. |
| Cell Assay | BON-1 cells (7,500 per well) and NCI-H727 cells (5,000 per well) are seeded into flat-bottomed 96-well plates in triplicate and allowed to adhere overnight in 10% fetal bovine serum-supplemented DMEM or RPMI 1640 complete medium, respectively; the medium is then exchanged for serum-free medium (negative control) or serum-free medium containing serial dilutions of Imatinib. After 48 h (control cultures do not reach confluence), the number of metabolically active cells is determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, and absorbance is measured in a Packard Spectra microplate reader at 540 nm. Growth inhibition is calculated. Experiments are done in triplicates[4]. |
| Animal Admin | Mice[5] The 40 tumor-bearing SCID mice are randomly divided into four groups (10 mice per group): the PS-ASODN group (5 μM, each mouse receives 0.2 mL by intratumor injection once daily); Imatinib group (0.1 mg/g body weight); liposome negative control group (0.01 mL/g); and saline group (0.01 mL/g). The mice in each group receive the relevant treatment by intra-tumor injection once daily from day 7 to day 28 after implantation. After 28 d, the mice are sacrificed, and tumor weight and longest and shortest diameters are measured by electronic scale and vernier caliper, respectively. Inhibition of tumor growth is calculated. Rats[6] Adult female Wistar-Albino rats (220-240 g) are used. Twenty-one days after the first surgical procedure, the rats undergo a second laparotomy to evaluate the occurrence of endometriosis. Twenty-four rats have visually confirmed endometriotic implants and are randomized into three groups to receive Imatinib (25 mg/kg/day, p.o.), Anastrozole (0.004 mg/day, p.o.), or normal saline (0.1 mL, i.p.) for 14 days. |
| References | [1]. Heinrich MC, et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000 Aug 1;96(3):925-32. [2]. Guida T, et al. Sorafenib inhibits imatinib-resistant KIT and platelet-derived growth factor receptor beta gatekeeper mutants. Clin Cancer Res. 2007 Jun 1;13(11):3363-9. [3]. Okuda K, et al. ARG tyrosine kinase activity is inhibited by STI571.Blood. 2001 Apr 15;97(8):2440-8 [4]. Yao JC, et al. Clinical and in vitro studies of imatinib in advanced carcinoid tumors. Clin Cancer Res. 2007 Jan 1;13(1):234-40. [5]. Sun XC, et al. Depletion of telomerase RNA inhibits growth of gastrointestinal tumors transplanted in mice. World J Gastroenterol. 2013 Apr 21;19(15):2340-7. [6]. Yildiz C, et al. Effect of imatinib on growth of experimental endometriosis in rats. Eur J Obstet Gynecol Reprod Biol. 2016 Feb;197:159-63. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 451°C |
| Melting Point | 113°C |
| Molecular Formula | C29H31N7O |
| Molecular Weight | 493.603 |
| Flash Point | 196°C |
| Exact Mass | 493.259003 |
| PSA | 86.28000 |
| LogP | 2.48 |
| Vapour Pressure | 6.03E-24mmHg at 25°C |
| Index of Refraction | 1.672 |
| Storage condition | Refrigerator |
| Hazard Codes | T,N |
|---|---|
| Risk Phrases | R23/24/25:Toxic by inhalation, in contact with skin and if swallowed . R40:Limited evidence of a carcinogenic effect. R48/23/24:Toxic: danger of serious damage to health by prolonged exposure through inhalation and in contact with skin . R51/53:Toxic to aq |
| Safety Phrases | S28-S36/37-S45-S61-S24/25-S23-S53 |
| RIDADR | UN 1662 6.1/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 6.1 |
| HS Code | 2933990090 |
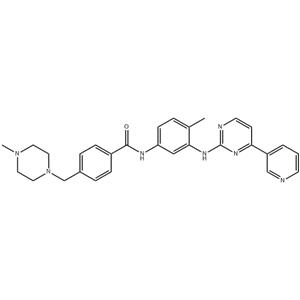
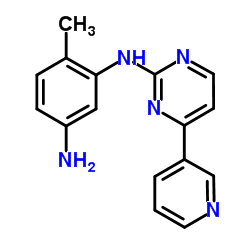 N-(5-Amino-2-me... 152460-10-1 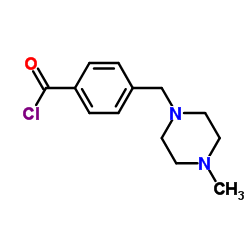 4-(4-Methylpipe... 148077-69-4 ~70% 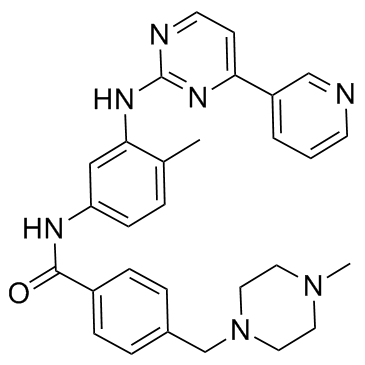 Imatinib (STI57... 152459-95-5 |
| Literature: CHEMAGIS LTD. Patent: EP1857454 A1, 2007 ; Location in patent: Page/Page column 4 ; |
 N-(5-Amino-2-me... 152460-10-1 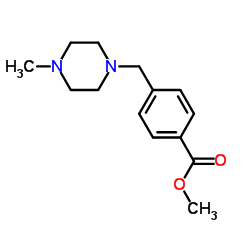 Methyl 4-[(4-me... 314268-40-1 ~90%  Imatinib (STI57... 152459-95-5 |
| Literature: Shen, Xin; He, Xiao; Yang, Jidong; Wu, Shaohong; Zhan, Huaxing Patent: US2013/41149 A1, 2013 ; Location in patent: Paragraph 0025 ; |
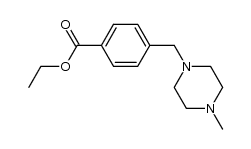 4-(4-methyl pip... 1044872-32-3  N-(5-Amino-2-me... 152460-10-1 ~92%  Imatinib (STI57... 152459-95-5 |
| Literature: Shen, Xin; He, Xiao; Yang, Jidong; Wu, Shaohong; Zhan, Huaxing Patent: US2013/41149 A1, 2013 ; Location in patent: Paragraph 0028 ; |
| Precursor 7 | Previous 1/2 Next |
|---|---|
| |
| DownStream 2 | |
| |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Benzamide, 4-[(4-methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]- (9CI) |
| Imatinib |
| STI571 |
| Imatinib (STI571) |
| Benzamide, 4-[(4-methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]- |
| 4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide |
| 4-[(4-methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide |
| 1iep |
| UNII-BKJ8M8G5HI |
| Benzamide, 4-((4-methyl)-1-piperazinyl)methyl)-N-(4-methyl-3-((4-(3-pyridinyl)-2-pyrimidinyl)amino)phenyl)- |
| MFCD05662257 |
| 4-[(4-Methyl-1-piperazinyl)methyl]-N-(4-methyl-3-{[4-(3-pyridinyl)-2-pyrimidinyl]amino}phenyl)benzamide |
| Imatinib free base |
| 1xbb |
| Name | s-allyl-l-cysteine |
|---|---|
| Synonym | More Synonyms |
| Description | S-Allyl-L-cysteine, one of the organosulfur compounds found in AGE, possess various biological effects including neurotrophic activity, anti-cancer activity, anti-inflammatory activity. |
|---|---|
| Related Catalog | Signaling Pathways >> Others >> Others Research Areas >> Cancer Research Areas >> Neurological Disease Natural Products >> Others Research Areas >> Inflammation/Immunology Research Areas >> Metabolic Disease Research Areas >> Others |
| In Vitro | It is found that S-Allyl-L-cysteine could protect against amyloid-protein (A)-and tunicamycin-induced cell death in differentiated PC12 cells. Simultaneously applied S-Allyl-L-cysteine (1 μM) suppresses the cell death induced by Aβ25-35 and Aβ1-40 in a concentration-dependent manner, and neuronal integrity is almost completely retained. Simultaneously applied S-Allyl-L-cysteine significantly decreases the Aβ-induced level of ROS. The TEAC value of S-Allyl-L-cysteine is lower than that of oxidized GSH, and no antioxidant activity is observed. Intracellular GSH levels remains unaffected by treatment of neurons with S-Allyl-L-cysteine for 24 h. Furthermore, the increase in caspase-12 protein expression is suppressed by simultaneously adding 1 μM S-Allyl-L-cysteine [1]. S-Allyl-L-cysteine up to a concentration 1.0 mM does not exhibit any cytotoxic impact on morphology of myoblast and myotubes in culture observed under bright field microscope. TNF treatment leads to a significant decrease in the intracellular CK activity while S-Allyl-L-cysteine pre-treatment to TNF treated myotubes decreases the release of CK in media. S-Allyl-L-cysteine pre-treatment decreases the level of active form of this enzyme in S-Allyl-L-cysteine+TNF group. Similar observations are recorded at mRNA level for caspase-3. These results illustrate that S-Allyl-L-cysteine regulates apoptotic signals via suppressing the transcription and thus protein expression of caspase-3[2]. |
| References | [1]. Kosuge Y, et al. S-allyl-L-cysteine selectively protects cultured rat hippocampal neurons from amyloid beta-protein- and tunicamycin-induced neuronal death. Neuroscience. 2003;122(4):885-95. [2]. Dutt V, et al. S-allyl cysteine inhibits TNFα-induced skeletal muscle wasting through suppressing proteolysis and expression of inflammatory molecules. Biochim Biophys Acta. 2018 Apr;1862(4):895-906. |
| Density | 1.191 |
|---|---|
| Boiling Point | 300 ºC |
| Melting Point | 235-236 ºC |
| Molecular Formula | C6H11NO2S |
| Molecular Weight | 161.222 |
| Flash Point | 135 ºC |
| Exact Mass | 161.051056 |
| PSA | 88.62000 |
| LogP | 1.31 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.543 |
| Storage condition | 2-8°C |
S-Allyl-L-cysteine MSDS(Chinese) |
| Symbol |  GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317 |
| Precautionary Statements | P280 |
| Hazard Codes | F,C |
| Risk Phrases | R11:Highly Flammable. R22:Harmful if swallowed. R34:Causes burns. R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . |
| Safety Phrases | S16-S26-S36/37/39-S45 |
| RIDADR | UN 2379 3/PG 2 |
| WGK Germany | 3 |
| RTECS | EO4460000 |
| Packaging Group | II |
| Hazard Class | 3.1 |
| HS Code | 2930909090 |
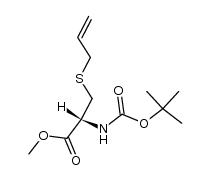 N-Boc-S-allyl-L... 232953-12-7 ~56% 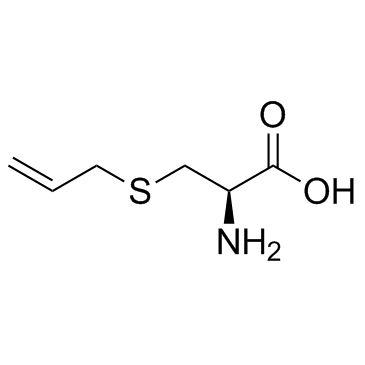 S-Allyl-L-cyste... 21593-77-1 |
| Literature: ISIS INNOVATION LIMITED Patent: US2012/178913 A1, 2012 ; Location in patent: Page/Page column 10-11 ; |
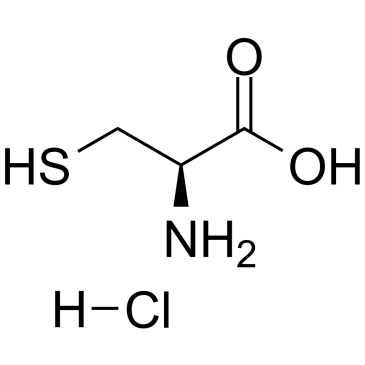 L-Cysteine hydr... 52-89-1 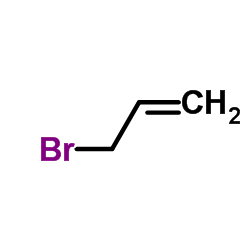 allyl bromide 106-95-6 ~70%  S-Allyl-L-cyste... 21593-77-1 |
| Literature: Maldonado, Perla D.; Alvarez-Idaboy, J. Raul; Aguilar-Gonzalez, Adriana; Lira-Rocha, Alfonso; Jung-Cook, Helgi; Medina-Campos, Omar Noel; Pedraza-Chaverri, Jose; Galano, Annia Journal of Physical Chemistry B, 2011 , vol. 115, # 45 p. 13408 - 13417 |
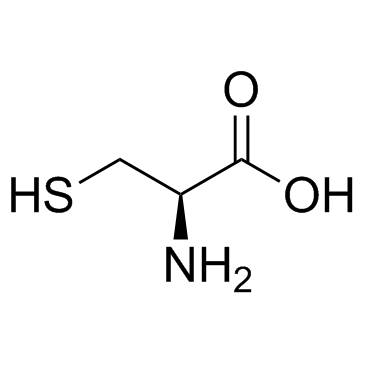 L-cysteine 52-90-4  allyl bromide 106-95-6 ~84%  S-Allyl-L-cyste... 21593-77-1 |
| Literature: Koch; Keusgen, Michael Pharmazie, 1998 , vol. 53, # 10 p. 668 - 671 |
| Precursor 10 | Previous 1/3 Next |
|---|---|
| |
| DownStream 9 | Previous 1/3 Next |
| |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
2. Packaging of materials
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping & Delivery
By Express
Provide door to door service
Suitable for goods under 50kg
Delivery: 3-7 days
Cost: low cost

By Air
Provide airport to airport service
Suitable for goods over 50kg
Delivery: 3-14 days
Cost: high cost

By Sea
Provide seaport to seaport service
Suitable for goods over 100kg
Delivery: 2-45 days
Cost: low cost

4. Contact information
For more details, pls contact us freely.
Email address: Sun@fdachem.com
Mob: 86 13526505137
WhatsApp/Skype/Wechat/LINE: 86 13526505137
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
Moxin Chemicals
|
2024-12-10 | |
| $10.00/1KG |
VIP6Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2024-11-28 | |
| $79.00/500mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $0.00/25KG |
VIP1Y
|
Shaanxi Dideu New Materials Co. Ltd
|
2024-11-13 | |
| $0.00/1kg |
VIP3Y
|
HangZhou RunYan Pharma Technology Co.,LTD.
|
2024-09-11 | |
| $19.10/1KG |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-20 | |
| $999.00/10kg |
VIP1Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-08 | |
| $0.00/1Gram |
VIP2Y
|
Hangzhou Hyper Chemicals Limited
|
2024-05-09 | |
| $0.00/1kg |
Nanjing Fred Technology Co., Ltd
|
2023-12-06 | ||
| $0.00/25Kg/Bag |
VIP4Y
|
Sinoway Industrial co., ltd.
|
2023-10-18 |
- Since: 2023-02-10
- Address: Room 01, 2288 E05, Building 14, East Henan University, Science and Technology Park, 279 Xisanhuan Ro




 CAS#:571186-91-9
CAS#:571186-91-9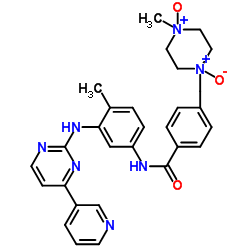 CAS#:571186-93-1
CAS#:571186-93-1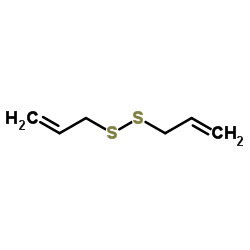 CAS#:2179-57-9
CAS#:2179-57-9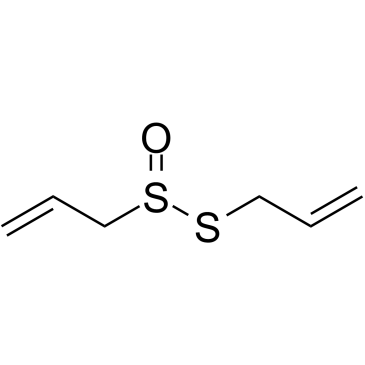 CAS#:539-86-6
CAS#:539-86-6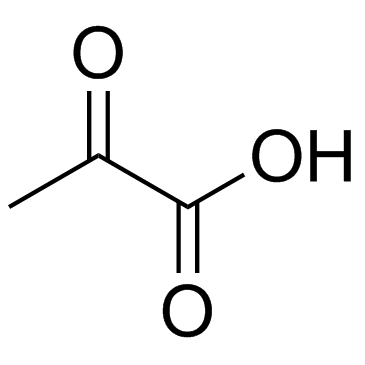 CAS#:127-17-3
CAS#:127-17-3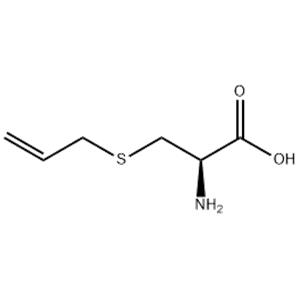

 China
China