Product Details
| Product Name:
trisethyleneiminoquinone |
CAS No.:
68-76-8 |
| Min. Order:
1g |
Purity:
96%-99% |
| Supply Ability:
1kg/10kg/100kg |
Release date:
2021/01/06 |
Amy 385 Email: amy@coreychem.com
Career Henan Chemical Co
Mob/Skype: +86-18317388371
| trisethyleneiminoquinone Basic information |
| Product Name: |
trisethyleneiminoquinone |
| Synonyms: |
1,1',1''-(3,6-dioxo-1,4-cyclohexadiene-1,2,4-triyl)tris aziridine;10257 R.P.;2,3,5-triethyleneimino-1,4-benzoquinone;2,3,5-tris(1-aziridinyl)-2,5-cyclohexadiene-1,4-dione;2,3,5-tris(1-aziridinyl)-p-benzoquinone;2,3,5-tris(aziridinyl)-1,4-benzoquinone;bayer 3231;oncovedex |
| CAS: |
68-76-8 |
| MF: |
C12H13N3O2 |
| MW: |
231.25052 |
| EINECS: |
200-692-6 |
| Product Categories: |
|
| Mol File: |
68-76-8.mol |
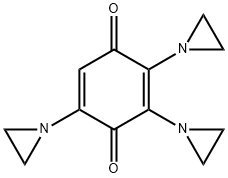 |
| |
| trisethyleneiminoquinone Chemical Properties |
| Melting point |
162.5-163° |
| Boiling point |
373.33°C (rough estimate) |
| density |
1.1818 (rough estimate) |
| refractive index |
1.5290 (estimate) |
| IARC |
3 (Vol. 9, Sup 7) 1987 |
| EPA Substance Registry System |
Triaziquone (68-76-8) |
| RIDADR |
3249 |
| HazardClass |
6.1(a) |
| PackingGroup |
II |
| |
| trisethyleneiminoquinone Usage And Synthesis |
| Originator |
Trenimon,Bayer |
| Uses |
Alkylating reagent in mutation research. |
| Definition |
ChEBI: A member of the class of 1,4-benzoquinones that is 1,4-benzoquinone in which three of the ring hydrogens are replaced by aziridin-1-yl groups. |
| Manufacturing Process |
Under anaerobic conditions including an atmosphere of nitrogen, 104 ml (2.0 moles) of aziridine is added as a single portion with stirring to a suspension of 33.6 g (0.2 mole) of 2,6-dimethoxy-1,4-benzoquinone in 500 ml of absolute methanol at a temperature of 0° to 5°C. After the addition has been 2,6- dimethoxy-1,4-benzoquinone completed the external cooling of the reaction vessel is replaced by a room temperature water bath and the mixture is stirred at room temperature for 45 hours while a slow stream of nitrogen is passed therethrough to preserve the anaerobic reaction conditions. It is found that the yellow starting material, during this period, completely disappears into solution and that a violet or purple substance together with a colorless substance are formed as precipitated reaction products. This mixture of precipitated products is removed by filtration at -20°C and the residue is washed with a small quantity of cooled methanol. Then the mixture is dried in a vacuum desiccator yielding about 30.4 g of mixed product. The mixed product is extracted with benzene whereby the violet or purple colored component passes into solution and the substantially colorless product remains in a yield of about 16.0 g. The colorless 2,6-bis-aziridino-l,4- benzohydroquinone so obtained melts with decomposition at about 221-222°C with melting starting at about 200°C. It can be purified by recrystallization from a of dioxane yielding snow-white crystals that decompose when heated at 222-224°C while starting to melt at 220°C. The benzene extract of the colored reaction product is evaporated to dryness under vacuum, yielding a residue melting at 161-162°C which is recrystallized from 200 ml of ethyl acetate, filtered under suction at -20°C, washed with cold methanol and thus yields about 11.5 g of a pure, purple-colored crystalline 2,3,5-tris-aziridino-1,4-benzoquinone melting at 162.5-163°C. |
| Therapeutic Function |
Antineoplastic |
| General Description |
Purple needle-like crystals. |
| Air & Water Reactions |
Slightly soluble in water. |
| Reactivity Profile |
trisethyleneiminoquinone is an amine. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. |
| Fire Hazard |
Flash point data is not available for trisethyleneiminoquinone, but trisethyleneiminoquinone is probably combustible. |
| Safety Profile |
Poison by intraperitoneal, intravenous, and parenteral routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental carcinogenic data. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx. Used as a drug for the treatment of neoplastic dseases. |
Company Profile Introduction
Technology research and transfer of pilot screening, pilot scale-up and mass production solutions , for pharmaceutical intermediates, biologicals and fine chemicals (Not including inflammable , explosive , hazardous goods ) ; Market : Nano material , metal catalyst , raw material of cosmetics , pharmaceutical intermediates , chemicals , photoelectric material , import and export of technology and goods (For projects subject to approval according to law, business activities can be carried out only after approval by relevant departments ).
You may like
-
CAS:158817-11-9
$1.80 / 1KG
-
CAS:58166-27-1
$1.80 / 1KG
-
CAS:852570-80-0
$1.80 / 1KG
Recommended supplier
| Product name |
Price |
|
Suppliers |
Update time |









 China
China