
Vonoprazan Fumarate
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 2KG |
| Supply Ability: | 20tons |
| Update Time: | 2024-07-26 |
Product Details
| Product Name: Vonoprazan Fumarate | CAS No.: 1260141-27-2 |
| Min. Order: 2KG | Purity: 99% min/GMP/PMDA/DMF |
| Supply Ability: 20tons | Release date: 2024/07/26 |
Product Information

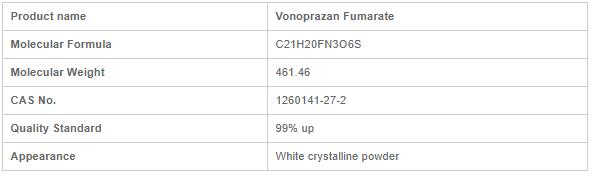
COA of Vonoprazan Fumarate
Usage
Function of Vonoprazan Fumarate
1) Prevention of duodenal ulcer and gastric ulcer recurrence
A randomized, double-blind, multi-center clinical phase 3 trial comparing the effects of Voronazan fumarate (10mgqd and 20mgqd) and lansoprazole (15mgqd) on nonsteroidal anti-inflammatory drug-related peptic ulcer A total of 642 patients had been diagnosed with endoscopic peptic ulcers and needed to take non-steroidal anti-inflammatory drugs. The treatment period was 24 weeks. The primary endpoint was the proportion of duodenal ulcer and gastric ulcer recurrence at 24 weeks.
2) Corrosive esophagitis (erosiveoesophagitis, EO)
In a randomized, double-blind, multi-center, dose-range clinical phase 2 trial, in patients with EO, compared with lansoprazole, Voronazan fumarate showed non-inferiority and was rated in Los Angeles as C/D grade patients showed excellent effects, and oral administration of 20 mg once daily became the clinically recommended dose for the treatment of EO. A randomized, double-blind, multi-center clinical phase 3 trial comparing the efficacy of this product (20mgqd) and lansoprazole (30mgqd) on EO, a total of 409 patients participated in the study.
3) Helicobacter pylori infection (Helicobacterpylori, Hp)
A randomized, double-blind, multi-center clinical phase 3 trial comparing vonolazan fumarate (20mgbid) and lansoprazole (30mgbid), combined with amoxicillin and clarithromycin, to form a triple therapy, The effect of first-line medicine to eradicate Hp was included in a total of 650 Hp-positive patients who had had gastric ulcer or duodenal ulcer. The Hp eradication rates of this product and lansoprazole in the test group were 92.6% and 75.9%, respectively. For patients with clarithromycin resistance, the Hp eradication rates in the two test groups were 82.0% and 40.0%, respectively. Significantly superior to lansoprazole. In this trial, the first 50 patients who failed first-line treatment received second-line treatment with triple therapy of this product, amoxicillin and metronidazole, and the Hp clearance rate was 98%.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $40.00/1box |
VIP1Y
|
Strong peptide cross-border e-commerce Co. LTD
|
2024-06-07 | |
| $10.00/1kg |
VIP1Y
|
Hebei Ganmiao New material Technology Co., LTD
|
2024-06-06 | |
| $15.00/1kg |
VIP1Y
|
Ouhuang Engineering Materials (Hubei) Co., Ltd
|
2024-04-24 | |
| $50.00/1kg |
VIP2Y
|
Zibo Hangyu Biotechnology Development Co., Ltd
|
2023-10-30 | |
| $35.00/1kg |
VIP1Y
|
Hebei Xinsheng New Material Technology Co., LTD.
|
2023-10-12 | |
| $30.00/1KG |
VIP2Y
|
Firsky International Trade (Wuhan) Co., Ltd
|
2023-10-08 | |
| $30.00/1kg |
Anhui Ruihan Technology Co., Ltd
|
2023-09-11 | ||
| $50.00/1KG |
VIP5Y
|
Hebei Mojin Biotechnology Co., Ltd
|
2023-08-15 | |
| $0.00/1g |
VIP2Y
|
shandong perfect biotechnology co.ltd
|
2023-08-03 | |
| $0.00/1kg |
VIP2Y
|
Hangzhou ICH Biofarm Co., Ltd
|
2023-07-17 |
- Since: 1996-07-16
- Address: Fujian China ,16F, Huicheng Comm. Complex, 839 Xiahe RD.











 China
China