
Lubiprostone NEW
| Price | Get Latest Price | ||
| Package | 10G/Bag | 100G/Bag | 1000G/Bag |
| Min. Order: | 1G/Bag |
| Supply Ability: | 2 tons |
| Update Time: | 2024-07-22 |
Product Details
| Product Name: Lubiprostone | CAS No.: 333963-40-9 |
| EC-No.: 1312995-182-4 | Min. Order: 1G/Bag |
| Purity: 99% up | Supply Ability: 2 tons |
| Release date: 2024/07/22 |
Product Information
Product name | Lubiprostone |
CAS No. | 333963-40-9 |
Molecular Formula | C20H32F2O5 |
Molecular Weight | 390.46 |
Molecular Structure | 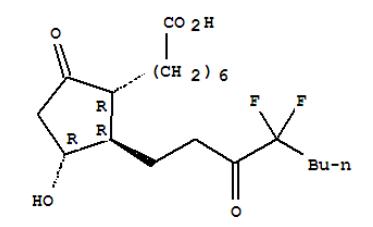 |
Quality Standard | 99% up by HPLC, for R&D use only |
Appearance | White powder |
COA of Lubiproston
Items | Standards | Results |
Appearance | White crystals or crystalline powder, odorless | Complies |
Identification | By IR | Complies |
By HPLC | Complies | |
Water Content | ≤ 0.50% | 0.22% |
Heavy Metals | ≤ 10ppm | Complies |
Residual Solvent | n-Hexane: ≤ 290ppm | ND |
Ethyl acetate: ≤ 5000ppm | 0.12% | |
Related Substances | Total impurities: ≤1.00% | 0.04% |
Purity(HPLC) | ≥ 99.0% | 99.96% |
Reference Standard | In-house Standard | |
Conclusion | The product complied to In-house standard. | |
Usage
Function of Lubiprostone
Lubiprostone is a limited chloride channel activator, which can selectively activate the type 2 chloride channel (CIC-2) located on the luminal cell membrane of the epithelial tip of the gastrointestinal tract to increase the secretion of intestinal juice and intestinal motility, thereby Increase bowel movements, reduce the symptoms of chronic idiopathic constipation, and do not change the concentration of sodium and potassium in the plasma.
Application of Lubiprostone
Adult chronic idiopathic constipation, constipation-type irritable bowel syndrome (only for female patients over 18 years old);
Company Information

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $1.00/1ASSAYS |
VIP5Y
|
Career Henan Chemical Co
|
2020-01-08 |
- Since: 1996-07-16
- Address: Fujian China ,16F, Huicheng Comm. Complex, 839 Xiahe RD.











 China
China