
Etoricoxib
| Price | $7 |
| Package | 1KG |
| Min. Order: | 1KG |
| Supply Ability: | 1000KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Etoricoxib | CAS No.: 202409-33-4 |
| Min. Order: 1KG | Purity: 99% |
| Supply Ability: 1000KG | Release date: 2019/07/06 |
JD607
| Product Name: | Etoricoxib |
| Synonyms: | Etoricoxib Solution, 100ppm;Etoricoxib (200 mg);Etoricoxib impurity A;Etoricoxib, >=98%;5-chloro-2-(6-methylpyridin-3-yl)-3-(4-methylsulfonylphenyl)pyridine;ETORICOXIB;5-Chloro-6'-methyl-3-[4-(methylsulfonyl) phenyl]-2,3'-bipyridine;Arcoxia |
| CAS: | 202409-33-4 |
| MF: | C18H15ClN2O2S |
| MW: | 358.84 |
| EINECS: | 1592732-453-0 |
| Product Categories: | Osteoarthritis and Rheumatoid Arthritis;Intermediates & Fine Chemicals;Pharmaceuticals |
| Mol File: | 202409-33-4.mol |
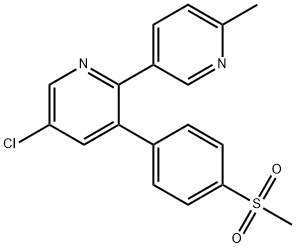 |
|
| Etoricoxib Chemical Properties |
| Melting point | 134-135°C |
| storage temp. | -20°C Freezer |
| pka | 4.5(at 25℃) |
| InChIKey | MNJVRJDLRVPLFE-UHFFFAOYSA-N |
| CAS DataBase Reference | 202409-33-4(CAS DataBase Reference) |
| Safety Information |
| Hazard Codes | T |
| Risk Statements | 22-24 |
| Safety Statements | 36/37-45 |
| RIDADR | UN 2811 6.1 / PGII |
| MSDS Information |
| Etoricoxib Usage And Synthesis |
| Cyclooxygenase -2 (COX-2) inhibitors | Etoricoxib is a kind of highly selective cyclooxygenase-2 (COX-2) inhibitors developed by the Merck company with the chemical name being 5-chloro-6'-methyl-3-4-(methanesulfonamide) phenyl]-2, 3'-bipyridine. Etoricoxib has a unique chemical structure that is methylsulfonyl group. The introduction of this group can not only increase the selectivity for COX-2 drugs, but also does not produce sulfa drugs and cross-allergic reactions. Etoricoxib was first approved for entering into market in 2002 in the UK, followed by the marketing countries and regions including the European Union, Asia, Australia and Latin America. Until the end of 2013, it has been approved for marketing in 97 countries for being widely used in treatment of osteoarthritis (OA), rheumatoid arthritis, ankylosing spondylitis, chronic low back pain, acute gouty arthritis, primary dysmenorrhea and postoperative pain, and other diseases. Etoricoxib has also entered into market in Taiwan and Hong Kong of China. It had entered into market in Chinese mainland in 2008 with the approved indications being acute gouty arthritis and OA and another indication being primary dysmenorrhea in the second half year of 2014. |
| Pharmacological effects | Etoricoxib is a non-steroidal anti-inflammatory drug with anti-inflammatory, analgesic and antipyretic effects in animal models. It is a kind of orally active, selective cyclooxygenase-2 inhibitor within the clinical dose range or higher doses. It has already confirmed of two subtypes of cyclooxygenase: cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). COX-1 is involved in the prostaglandin mediated normal physiologic functions such as gastric cytoprotection and platelet aggregation. Non-selective non-steroidal anti-inflammatory drugs inhibit the generation of COX-1, and thus can cause gastric mucosal injury and decreased platelet aggregation. COX-2 is mainly involved in the production of prostaglandins, and prostaglandins can cause pain, inflammation and fever. Etoricoxib is a kind of selective COX-2 inhibitors which can reduce these symptoms and signs as well as reduce gastrointestinal side effects without affecting platelet function. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Pharmacokinetics | Absorption: it has excellent oral absorption with the mean oral bioavailability being close to 100%. For adult, it can be subject to oral administration of 120mg upon empty stomach for once daily until it reaches steady state. Its plasma concentration reaches peak in about 1 hour after drug administration. This pharmacokinetic of this product exhibits linear correlation within clinical dose range. Distribution: in the concentration range 0.05-5mcg/ml; 92% binds to human plasma protein. In the human body, volume of distribution at steady state is approximately 120 liters. It can penetrate the placenta of rats and rabbits as well as the blood-brain barrier in rats. Metabolism: Metabolic is complete with the proto-drug content in the urine being less than 1%. The main metabolic pathway is through the catalysis through the enzyme cytochrome P450 (CYP), forming a six-carboxylic acid derivative. Clearance: In healthy individuals, intravenous administration of radio-labeled Etoricoxib; 70% of the radioactivity could be detected in the urine, 20% of the radioactivity could be detected in the feces while most of them being in the form of the presence of metabolites and only less than 2% of the prototype drug is excreted. |
| Drug Interactions | Warfarin: long-term usage of warfarin therapy in patients with stable efficacy. Daily application of this drug should be 120mg with the prothrombin time being approximately 13% higher than the international normalized ratio (INR). Rifampicin: Rifampicin is a strong inducer of hepatic metabolism. The combination of this product with rifampicin allows the plasma area under the curve (AUC) to be reduced by 65%. Therefore when this product is combined with rifampin, we should take into account their interaction. Methotrexate: When using this product at a dose greater than 90mg/day as well as being in combination with methotrexate, you should consider monitoring the toxicity associated with methotrexate. Diuretics, angiotensin-converting enzyme (ACE) inhibitors and angiotensin II antagonists (AIIAs): Non-steroidal anti-inflammatory drugs, including selective cyclooxygenase-2 inhibitors can reduce the antihypertensive effect of the diuretics, angiotensin converting inhibitors and angiotensin II antagonists. Lithium salt: non-selective non-steroidal anti-inflammatory drugs and cyclooxygenase-2 selective inhibitors may increase plasma levels of lithium salts. Aspirin: It can be used simultaneously with a lose-dose aspirin for the prevention of cardiovascular events. However, upon being in combination with low-dose aspirin, the incidence of gastrointestinal ulcers or other complication rate is higher than in the case of single usage of this product. Oral contraceptives: upon selecting suitable oral contraceptives for being used in combination with this product, we need to take into account of the increase of the EE concentration. The increase of the EE concentration will increase the incidence of the related adverse events of oral contraceptives (such as the risk of venous thromboembolism for women). Other: Antacids and ketoconazole (CYP3A4 strong inhibitors) do not produce clinically significant impact on the pharmacokinetics of this product. |
| Indications | 1. the treatment of OA: the good has a comparable effect as celecoxib, ibuprofen with similar efficacy with large doses of diclofenac and naproxen. 2. acute gouty arthritis: This product is 120 mg/d and can quickly and effectively relieve pain, and has a similar efficacy as the gold standard drug indomethacin in the treatment of gouty arthritis with a better tolerance and a lower incidence of drug-related adverse events than indomethacin. 3. ankylosing spondylitis: etoricoxib has a similar efficacy as naproxen taken twice per day; etoricoxib has a better effect in treating secondary end-point aspects such as alleviating night pain, inflammation, functionality and flexibility. 4. rheumatoid arthritis: large-scale, controlled clinical trial results showed that etoricoxib taken once daily has a better efficacy compared to naproxen taken twice daily with a better tolerance in patients. 5. osteoarthritis: This product (once/day) has a comparable efficacy as celecoxib (once/day), and ibuprofen (3 times/day); and it (once/day) has a similar efficacy with high-dose diclofenac (3 times/day) and naproxen (3 times/day). 6. postoperative dental pain: compared with acetaminophen/codeine and oxycodone/ p-acetaminophen, taking etoricoxib once daily can yield a better efficacy in alleviating the pain feeling of patients. 7. chronic low back pain: compare this product with Placebo on the treatment efficacy of chronic low back pain in 12 weeks has demonstrated that the clinical efficacy of this product was significantly superior to placebo drug with being effective in the treatment for only one week while the effect is very significant in four weeks and the sustained effect being able to reach over three months. 8. for controlling late pain of postoperative thyroid: when thyroid operation patients orally take etoricoxib at 1 h before operation can reduce the oral administrated dose of oxycodone acetaminophen at 6~12 h after surgery in patients. |
| Safety | Compared with diclofenac, the incidence of this product in inducting thrombotic cardiovascular events has no significant difference while the cumulative incidence of clinically diagnosed gastrointestinal perforation, ulcers, bleeding in etoricoxib group was significantly lower than the diclofenac group. The incidence of gastrointestinal adverse events is decreased by 50% compared with the diclofenac sodium. Etoricoxib has a low gastrointestinal reaction. During the treatment, the gastrointestinal symptoms (nausea, vomiting, abdominal pain or discomfort, diarrhea), chest and ankle edema and other adverse events for etoricoxib is similar to other selective COX-2 inhibitors. It is contraindicated in patients with ischemic heart disease and stroke. For patients with risk factors such as heart disease, they should use with caution. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $100.00/1kg |
VIP3Y
|
Hebei Duling International Trade Co. LTD
|
2024-05-21 | |
| $0.00/25kg |
VIP1Y
|
PUSHAN INDUSTRIAL (SHAANXI) CO.,LTD
|
2024-05-13 | |
| $0.00/25KG |
VIP5Y
|
Hebei Mojin Biotechnology Co., Ltd
|
2023-08-28 | |
| $15.00/1kg |
Hebei Shengyuan Jinlong Import and Export Co. LTD
|
2022-10-31 | ||
| $157.00/1KG |
VIP4Y
|
Hebei Lingding Biotechnology Co., Ltd.
|
2022-03-04 | |
| $9.00/1KG |
VIP4Y
|
Hebei Guanlang Biotechnology Co,.LTD
|
2022-01-21 | |
| $10.00/1Kg/Bag |
Hebei Zhanyao Biotechnology Co. Ltd
|
2021-11-01 | ||
| $0.00/1Kg/Bag |
VIP4Y
|
WUHAN FORTUNA CHEMICAL CO., LTD
|
2021-10-18 | |
| $9.90/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2021-09-28 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com




 China
China