
Tetramethylammonium hydroxide
| Price | $1 |
| Package | 1kg |
| Min. Order: | 1g |
| Supply Ability: | 100KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Tetramethylammonium hydroxide | CAS No.: 75-59-2 |
| Min. Order: 1g | Purity: 99% |
| Supply Ability: 100KG | Release date: 2019/07/06 |
| PRODUCT:: AD68 |
| Tetramethylammonium hydroxide Basic information |
| Product Name: | Tetramethylammonium hydroxide |
| Synonyms: | N,N,N-TRIMETHYLMETHANAMINIUM HYDROXIDE;TETRAMETHYLAMMONIUM HYDROXIDE;TMAH;hydroxydedetetramethylammonium;megapositcd14;Methanaminium,N,N,N-trimethyl-,hydroxide;n,n,n-trimethyl-methanaminiuhydroxide;Tetramethylammonium hydroxide concentrate |
| CAS: | 75-59-2 |
| MF: | C4H13NO |
| MW: | 91.15 |
| EINECS: | 200-882-9 |
| Product Categories: | quarternary ammonium bases;Ammonium Hydroxides (Quaternary);Quaternary Ammonium Compounds;Analytical Chemistry;Esterification & Alkylation (GC Derivatizing Reagents);GC Derivatizing Reagents;Onium Hydroxides (GC Derivatizing Reagents);ACS GradeChemical Synthesis;Essential Chemicals;Organic Bases;Routine Reagents;Synthetic Reagents;Electronic Chemicals;Micro/Nanoelectronics;Semiconductor Grade Chemicals;Chemical Synthesis;Reference Material Hydrochloric acidTitration;By Reference Material;Solutions for non-aqueous titrations;Volumetric Solutions;Base ConcentratesConcentrates (e.g. FIXANAL);Concentrates (e.g. FIXANAL);Reference Material Hydrochloric acid;Titration;Reference Material Perchloric acidTitration;Base SolutionsVolumetric Solutions;Organic BasesEssential Chemicals;Reagent Grade;Atomic Absorption Spectroscopy (AAS);Digestion Reagents;Pure Reagents for Wet Digestion (Trace SELECT);AnionicHPLC;Chromatography/CE Reagents;Ion Pair;Ion Pair Reagents;Ion Pair Reagents - Anionic Concentrate;NULL |
| Mol File: | 75-59-2.mol |
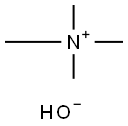 |
|
| Tetramethylammonium hydroxide Chemical Properties |
| Melting point | °C |
| Boiling point | 110 °C |
| density | 0.866 g/mL at 25 °C |
| vapor pressure | 17.5 mm Hg ( 20 °C) |
| refractive index | n20/D 1.384 |
| Fp | 80 °F |
| storage temp. | 2-8°C |
| form | Solution |
| color | APHA: ≤10 |
| Water Solubility | Soluble in water. |
| Sensitive | Air Sensitive |
| Merck | 14,9224 |
| BRN | 3558708 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, strong acids. |
| InChIKey | WGTYBPLFGIVFAS-UHFFFAOYSA-M |
| CAS DataBase Reference | 75-59-2(CAS DataBase Reference) |
| EPA Substance Registry System | Methanaminium, N,N,N-trimethyl-, hydroxide(75-59-2) |
| Safety Information |
| Hazard Codes | C,T,F,T+,Xn |
| Risk Statements | 24/25-34-20/21/22-39/23/24/25-23/24/25-10-21/22-25-21-11-67-36-52-48/24-27/28-23-35-36/38 |
| Safety Statements | 45-36/37/39-26-16-36/37-28 |
| RIDADR | UN 3286 3/PG 2 |
| WGK Germany | 1 |
| RTECS | PA0875000 |
| F | 4.4-10-34 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | II |
| HS Code | 29239000 |
| MSDS Information |
| Provider | Language |
|---|---|
| Tetramethylammonium hydroxide | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Tetramethylammonium hydroxide Usage And Synthesis |
| Chemical Properties | colourless to yellow liquid | ||||||||||||||||||||||||||
| Hazard | Strong irritant to skin and tissue. | ||||||||||||||||||||||||||
| Safety Profile | Poison by subcutaneous route. A powerful caustic. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of NOx and NH3. | ||||||||||||||||||||||||||
| Purification Methods | It is freed from chloride ions by passage through an ion-exchange column (e.g. Amberlite IRA-400, prepared in its OH-form by passing 2M NaOH until the effluent is free from chloride ions, then washed with distilled H2O until neutral). A modification, to obtain carbonate-free hydroxide, uses the method of Davies and Nancollas [Nature 165 237 1950]. [Beilstein 4 IV 145.] | ||||||||||||||||||||||||||
| Description | Tetramethylammonium hydroxide is a solid in the hydrated form or a colorless liquid with a strong ammonia-like odor. It is soluble in water, and corrosive to metals and tissue. | ||||||||||||||||||||||||||
| Uses |
|
||||||||||||||||||||||||||
| Health Hazard | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | ||||||||||||||||||||||||||
| Fire Hazard | Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. | ||||||||||||||||||||||||||
| Reactivity Profile | Tetramethylammonium hydroxide acts like a base. Bases are chemically similar to sodium hydroxide (NaOH) or sodium oxide (Na2O). They neutralize acids exothermically to form salts plus water. When soluble in water they give solutions having a pH greater than 7.0. Mixing these materials with water can generate troublesome amounts of heat as the base is dissolved or diluted. Bases react with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. Bases may initiate polymerization reactions in polymerizable organic compounds, especially epoxides). They may generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. Materials of this group often serve as catalysts. | ||||||||||||||||||||||||||
| References | 1.https://en.wikipedia.org/wiki/Tetramethylammonium_hydroxide#Uses 2.http://sacheminc.com/other-chemicals/tetramethylammonium-hydroxide-tmah/ 3.https://www.fs.usda.gov/treesearch/pubs/15506 4.https://www.concordia.ca/content/dam/concordia/services/safety/docs/EHS-DOC-020_TMAHGuidelines.pdf 5.https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2854236.htm |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1kg |
VIP5Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-09-13 | |
| $0.00/25KG |
VIP5Y
|
Hebei Mojin Biotechnology Co., Ltd
|
2023-09-06 | |
| $10.00/1kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-08-02 | ||
| $5.00/200KG |
VIP6Y
|
Jinan Finer Chemical Co., Ltd
|
2023-02-06 | |
| $10.00/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2022-12-30 | |
| $30.00/1KG |
VIP2Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2022-09-13 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $0.00/1KG |
VIP5Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-01-13 | |
| $6.00/6kg |
Chemwill Asia Co.,Ltd.
|
2019-04-04 | ||
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-08 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com





 China
China