
Cediranib
| Price | $2 |
| Package | 1kg |
| Min. Order: | 1kg |
| Supply Ability: | 100kg |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Cediranib | CAS No.: 288383-20-0 |
| Min. Order: 1kg | Purity: 99% |
| Supply Ability: 100kg | Release date: 2019/07/06 |
| Product Number:: WM.819 |
| Cediranib Basic information |
| Anticancer drugs |
| Product Name: | Cediranib |
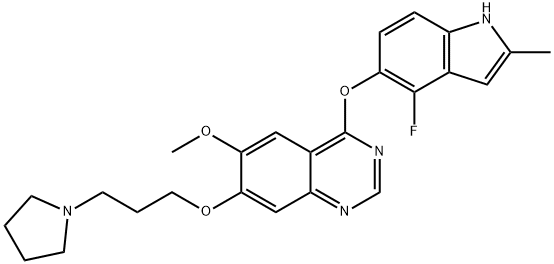
| Synonyms: | Cediranib;4-(4-Fluoro-2-methylindol-5-yloxy)-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy]quinazoline;Cadiranib (AZD2171);Cediranib(AZD2171);AZD2171;Recentin;Cadiranib;Cediranib R |
| CAS: | 288383-20-0 |
| MF: | C25H27FN4O3 |
| MW: | 450.51 |
| EINECS: | 1308068-626-2 |
| Product Categories: | APIs;Antineoplastic;Inhibitors |
| Mol File: | 288383-20-0.mol |
 |
|
| Cediranib Chemical Properties |
| density | 1.285 |
| Safety Information |
| MSDS Information |
| Cediranib Usage And Synthesis |
| Anticancer drugs | Cediranib is an oral anticancer drug , it is successfully developed by the US AstraZeneca company , its trade name is recentin, this product is an efficient VEGFR tyrosine kinase inhibitor, it is capable of inhibiting all known VEGFR tyrosine kinases ( including VEGFR-1, VEGFR-2, VEGFR-3, and PDGFR). Cediranib can inhibit VEGF-induced angiogenesis, neovascularization survival and human tumor xenograft cell growth.Clinical trail phase I for non-small cell lung cancer (abbreviated NSCLC) patients shows that cediranib in combination with PC programs has a significant antitumor effect . Clinical trail phaseⅡ/Ⅲ BR24 focuses on using cediranib 30mg as a first-line treatment of NSCLC ,which demonstrates that it has anti-tumor effect, but it has a higher incidence of adverse events, including diarrhea, dehydration, hand-foot syndrome, hypertension and neutropenia disease. Therefore, there is the decision to stop clinical trials. Repeated assessments about toxicity and dose of the combination of drugs suggest that part of the adverse effects are dose-related , dose reduction may be tried to improve drug tolerance. In view of this conclusion, in 2009, the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) launched a cediranib clinical trail phase Ⅲ (BR29) for progressive NSCLC patients , the dose was adjusted to 20mg. The results have not been reported. In 2009 ,European Society for Medical Oncology (ESMO) Annual report which is about cediranib treatment of metastatic renal cell carcinoma (referred mRCC) clinical trail phase II shows that cediranib treatment group tumor volume reducing rate is higher than the placebo group. Zurita tested Cediranib pretreatment serum cytokines and angiogenic factors ,and found that patients having low IL-10, VEGF, PIGF, SCF and MIG concentration during baseline time , have the more obvious tumor volume reduction after treatment. The above information is edited by the chemicalbook of Tian Ye. |
| Uses | Cediranib (AZD2171) inhibited VEGF-stimulated proliferation and KDR phosphorylation with IC50 of 0.4 and 0.5 nM, respectively. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $35.00/2mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $10.00/1KG |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-11-05 | |
| $0.00/25KG |
VIP5Y
|
Hebei Mojin Biotechnology Co., Ltd
|
2023-09-06 | |
| $12.00/1KG |
Hong Kong Tiansheng New Material Trading Co., Ltd
|
2022-01-10 | ||
| $1.00/1g |
Wuhan Boyuan Import & Export Co., LTD
|
2021-08-12 | ||
| $1.10/1g |
VIP4Y
|
Dideu Industries Group Limited
|
2021-07-01 | |
| $0.00/1Kg |
VIP6Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2020-04-30 | |
| $5.99/1KG |
Hong Kong Tiansheng New Material Trading Co., Ltd
|
2021-12-24 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com






 China
China