
Brinzolamide
| Price | $2 |
| Package | 1kg |
| Min. Order: | 1kg |
| Supply Ability: | 100kg |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Brinzolamide | CAS No.: 138890-62-7 |
| Min. Order: 1kg | Purity: 99% |
| Supply Ability: 100kg | Release date: 2019/07/06 |
| Product Number:: WM.819 |
| Brinzolamide Basic information |
| Ophthalmic drugs Brinzolamide eye drops Dosage Side effects Uses Production methods |
| Product Name: | Brinzolamide |
| Synonyms: | H-Thieno[3,2-e]-1,2-thiazine-6-sulfonamide, 4-(ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-, 1,1-dioxide, (4R)-;Brinzolamide (R)-4-(Ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-2H-thieno[3,2-e]-1,2-thiazine-6-sulfonamide 1,1-dioxide;(5R)-5-Ethylamino-3-(3-methoxypropyl)-2,2-dioxo-2?6,9-dithia-3-azabicyclo[4.3.0]nona-7,10-diene-8-sulfonamide;ANTI-GAGE7 (C-TERM) antibody produced in rabbit;Cancer/testis antigen 4.7;CT4.7;G antigen 7;GAGE-12I |
| CAS: | 138890-62-7 |
| MF: | C12H21N3O5S3 |
| MW: | 383.51 |
| EINECS: | 1308068-626-2 |
| Product Categories: | Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Brinzolamide;BETOPTIC |
| Mol File: | 138890-62-7.mol |
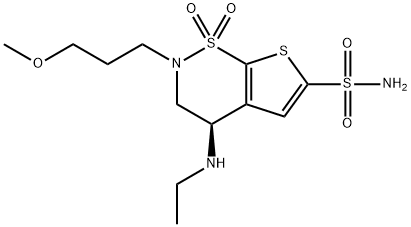 |
|
| Brinzolamide Chemical Properties |
| Melting point | 130.0 to 134.0 °C |
| storage temp. | ?20°C |
| solubility | DMSO: ≥10mg/mL |
| color | white to beige |
| Merck | 14,1376 |
| InChIKey | HCRKCZRJWPKOAR-JTQLQIEISA-N |
| CAS DataBase Reference | 138890-62-7(CAS DataBase Reference) |
| Safety Information |
| RTECS | XJ9095055 |
| MSDS Information |
| Brinzolamide Usage And Synthesis |
| Ophthalmic drugs | Brinzolamide is a kind of novel heterocyclic sulfa topical carbonic anhydrase inhibitor. It has a strong affinity and inhibition on the carbonic anhydrase isoenzyme II (CAII) in human ciliary body. It can selectively and significantly inhibit the activity of isozyme II carbonic anhydrase with high-affinity. It can effectively reduce the intraocular pressure with high efficiency, low toxicity, and small side effects. Its physiological pH and suspension design makes the drug able to ensure eye comfort and thus admired by patients of all ages. It can be as a single drug treatment way for patients to whom β-blockers is ineffective or is contraindicated or used as a co-cure treatment drug together with β-blocker. After being dropped into the eye it can quickly enter into tissue, and has a long half-life in the iris, ciliary body, choroid, retina, and lens as well as in the blood. Treating rabbit eyes with brinzolamide can further increase blood flow to the optic nerve which helps patients of glaucoma optic neuropathy. It has become the primary way for treatment of open angle glaucoma line drugs. Carbonic anhydrase (CA) is one of the major protein components of red blood cells. Its position is only second to hemoglobin in the blood cell, which is presented in many body tissues including the eye organizations. Carbonic anhydrase catalyzed hydration reaction of carbon dioxide into carbonic acid, and the reversible dehydration reaction of carbonic acid. Inhibition of carbonic anhydrase in the ocular ciliary body can reduce the water secretion of camera aquosa. It reduces the transport of sodium and water possibly by reducing the generation of bicarbonate ions, and ultimately reduces the intraocular pressure. IOP is an important risk factor for glaucoma optic nerve damage and glaucomatous visual field defects. Brinzolamide mainly inhibits the dominant carbonic anhydrase type 2 isoenzyme in the eye tissue. In vitro tests had shown that the IC50 was 3.2nM and the Ki value ??to the carbonic anhydrase type-II isoenzyme was 0.13 nM. |
| Brinzolamide eye drops | Brinzolamide eye drops (BrinzolamideEye Drops) is currently a good drug in the treatment of ocular hypertension and open-angle glaucoma remission within elevated intraocular pressure. In April 1998, it had entered into market for the first time in the United States, and has currently entered into market in nearly 30 countries. It was approved for import in China in 2002. It can be as a single drug treatment way for patients to whom β-blockers is ineffective or is contraindicated or used as a co-cure treatment drug together with β-blocker. Its brand name is Azopt. Brinzolamide eye drops is a sulfa drug, although used as eye drops, but can still be absorbed systemically. Therefore, adverse reactions of sulfa drugs may still occur in the eye drops with time. If severe adverse reactions or allergies occur, you should immediately stop using eye-drops. For patients subject to oral administration of carbonic anhydrase inhibitors or eye-drop of Azpot, there may be known systemic adverse reactions which are related with carbonic anhydrase inhibition. There have been no studies concerning the co-orally administration of carbonic anhydrase inhibitor and Azopt, thus it is not recommended to use both drugs in combination. Currently there is only limited experience on brinzolamide’s treatment of pseudo-capsular exfoliative and pigmentary glaucoma. In the combination therapy of glaucoma, people mainly evaluate the effects of brinzolamide and timolol. Therefore, information on joint use and other anti-glaucoma drugs is limited. There have been no studies on the effects of brinzolamide eye drops in patients with acute angle-closure glaucoma and severe renal impairment. Owing to that the product and most of its major metabolites are mainly excreted by the kidney, so those two kinds of patients are not recommended. Benzalkonium chloride is a commonly used ophthalmic preservative, and it has been reported to cause the punctate keratopathy or (and) ulcerative keratopathy. Azopt contains benzalkonium chloride as a preservative. It can be absorbed by soft contact lenses so it is necessary to wear contact lens only at 15 minutes after being subject to Brinzolamide eye drops. It is not allowed to apply its eye-drop at the same time of wearing contact lenses. Patients of diabetics, patients with dry eye, corneal lesion need to be closely observed. Pregnant and lactating women or children less than 18 years-old should use with caution. The above information is edited by the Chemicalbook of Dai Xiongfeng. |
| Dosage | Shake uniformly before using. As a monotherapy or co-therapy, drop one drop into the conjunctival sac 2 times per day; 3 times a day may be better for some patients. When use it as a substitute of another anti-glaucoma drug. Stop using that drug first and start using brinzolamide eye drops in the next day. Treatment with more than one drug should be separated by at least five minutes. It is generally recommended that oppress nasolacrimal duct or gently close your eyes after the medication in order to reduce systemic absorption. |
| Side effects | According to reports, the incidence of adverse reactions which is or may be related with brinzolamide eye drops were less than 10% commonly, and less than 1% rarely in clinical studies. In clinical tests of more than 1500 patients, when brinzolamide eye drops was either used alone or in combination with the use of timolol (0.5%), the most common treatment-related adverse reactions and local symptoms include: changes in taste (mouth pain and odor) (5.3%), transient blurred vision after treatment which lasts a few seconds to a few minutes (4.8%). It is reported in 1% to 5% of cases about pharyngitis, dermatitis, eye, foreign body sensation, headache, congestion, eye discharge, eye irritation, keratitis, eye pain, ocular pruritus and rhinitis. Very rare serious side effects include stomach pain, dark urine, easy bruising or bleeding, vision changes, persistent sore throat, and fever. Brinzolamide ophthalmic solution is contraindicated for people may be allergic to it. |
| Uses | It is carbonic anhydrase inhibitors suitable for treating high intraocular pressure or open-angle glaucoma. |
| Production methods | Thiourea (1.287kg, 16.93mo1), benzyl chloride (1.858L, 2.044 kg, 16.14mo1), 13.5 L of ethanol and 4.5 L of water, reflux for 2 h. Cool to 74 °C in 20 min. Followed by adding 3-acetyl-2,5-dichloro-thiophene (3.0 kg, 15.38 mol) and 10L4 mol/L aqueous sodium hydroxide. Reflux for 3 h until completion of reaction, cooled to room temperature overnight. Add 10 L of water, stir for 30min, then add 3L 5.25% sodium hypochlorite solution, stir again for 30min. The solid was collected by filtration and washed with 4×2.5 L of water and 3×2L isopropanol. Dry in air at room temperature to constant weight to give 4.224 kg compound (I), 97% yield; its m.p. 86~88 °C. Compound (I) (1kg, 3.53mo1) was dissolved in 20 L ethyl acetate, stir at 2~10 °C, pour chlorine gas until no Compound (I) detection. Blow a large air flow for 1h, then pour ammonia gas and then, keep the temperature 5~15 °C, keep the reaction until the intermediate thionyl chloride being complete converted. Then further use air for blowing 1h, add 5L of water cooled to 15 °C. Add sodium tungstate trihydrate (583g, 1.77mo1), further add 8 L 30% hydrogen peroxide in 5min. The mixture is stirred for 2 h at 35 °C, and further stirred at room temperature for 16 h; add 5L of water and separate the organic layer, and then add 5L of water again. Sodium bisulfite is added until no detectable hydroperoxide; separate the organic layer; wash with saturated sodium bicarbonate to PH value of 8; then wash with saturated NaCl solution, dry, filter, and concentrate; add tert-butyl methyl ether to the residue; filter to collect the solid; wash with tert-butyl methyl ether, and dry it in air to constant weight to yield Compound 597g (II), yield(71%), m.p. 178~179 °C. Compound (II) (1.087kg, 4.55mo1) was dissolved in 22L of ethyl acetate; cool in an ice bath; add 90% hydrogen bromide-bromopyridine through anchor (1.305kg, 3.67mo1); 544 mL sulfuric acid is added in 10min and the temperature was raised to 5 °C. After stirring for 1 h until the reaction is completed, stir again for 30min; add 5L of water and stir for 5min; then separate the organic layer and washed with 4 × 5L saturated brine to until the Ph value of 3. Dry, filter, and concentrate; add 2L of methylene chloride to the residue, freeze for 15min. The solid was collected by filtration, air dry at room temperature to a constant weight to give 1.041kg compound (III), yield:72%, mp 147~148 °C. Under the protection of nitrogen, add compound III (4855g, 2.68mo1) and 12.5 L tert-butyl methyl ether to the reaction flask, stir and cool to-40 °C. Within 30 min, add 4.5L 1.2mol/L of alkyl tert-butyl methyl ether solution of (+)-β-chloro-diisocyanate loose camphene boron. Stir at-25 ~-20 °C for 3.5h until the completion of the reaction, raise the temperature to 0 °C, added 11L 1mol/L aqueous sodium hydroxide solution in 10 min so that the temperature is raised to 22 °C. Stir vigorously at room temperature for 2 h to separate the organic layer; the aqueous layer is extracted with 3L tert-butyl methyl ether with the remaining aqueous layer being acidified with concentrated hydrochloric acid to a Ph value 1; extract it with 2 × 4 L ethyl acetate; ethyl acetate extract was washed with 3L brine, dry, filter, and concentrate. Add 2L of toluene and the crystals are further collected by filtration; wash it with 2L of toluene, then further wash with 2L of methylene chloride; dry it in air at room temperature to constant weight to give 498 g Compound IV), yield. 77%, melting point 126~127 °C. Compound (IV) (5350g, 1.46mo1), 1.75L dimethyl sulfoxide and potassium carbonate (605g, 4.38mo1) are mixed and separated into 8 parts with each part being added 1-bromo-3-methoxy propane (268g, 1.75mo1) at a interval of 1h. After the completion of the addition, it takes 1.5 h for the completion of the reaction. Under stirring, pour the reaction solution into a saturated 18 L saline with the original reactor washed with water and tert-butyl methyl ether wash. The reaction liquid and washings are combined and further extracted with 2×4L tert-butyl methyl ether. The extract is further washed by 2 L 1mol/L aqueous sodium hydroxide solution, 2L 1: 1 sodium hypochlorite/water, and 2L brine. Filter and evaporate most of the solvent, then concentrate under reduced pressure at 50 deg. to get 427g compound (V), 94% yield, as light yellow oil. Under the protection of nitrogen, the compound (V) (1.065kg, 3.42mo1) is added into 27 L dry tetrahydrofuran and cooled to-70 °C; Within 2.5h, add drop wise 3.08 L of 2.5 mol/L hexane solution of n-butyllithium. Maintain the reaction mixture temperature below-66 °C. After 1 h of the reaction, sulfur dioxide is passed into until the Ph value of 4. The temperature is raised to room temperature; keep the mixture stand overnight. Concentrate and dissolve the residue in 5L of water. Add it into sodium acetate trihydrate (0 °C, 2.796kg, 20.5mo1) and hydroxylamine-O-sulfonic acid (1.549kg, 13.7mo1) and dissolve in 6L of water, and increase the temperature to 25 °C. After stirring at room temperature for 15h, 3 × 4L ethyl acetate is used for extracting it. The extract is first washed with saturated sodium bicarbonate solution until the washing becomes basic, then further wash with saturated brine. Dry, filter, and concentrate. Add 6 L methylene chloride to the residue and further add 5g seed for crystallization. The crystals are collected by filtration, washed with dichloromethane, and dried with air at room temperature to constant weight to get 748g compound VI) with a yield of 61%, m.p.111~113 °C. Compound (VI) (28.5g, 0.08 mo1), 285ml of acetonitrile and 23.4ml trimethyl orthoacetate are mixed and refluxed for 16h. Cool for 1h and distill off the solvent with the residue being dissolved in 150ml of dry tetrahydrofuran. Under the protection of nitrogen, add 24.5ml triethylamine and 30.5g p-toluenesulfonyl chloride at 4°C and stir at 4~7 °C for 2 h. Add drop wise 70% solution of triethylamine (260ml, 2.80mo1) within 30min while maintaining the temperature below 15 °C. After addition, the mixture is further stirred at room temperature for 18.5h. Cool to 5 °C, and add drop wise 280 mL concentrated hydrochloric acid within 1 hour while maintaining the temperature below 30 °C. Extract with 2 × 250ml of ether and further extract with 200 mL 1mol/L hydrochloric acid. Hydrochloric acid extract is adjusted to pH 8 with solid sodium bicarbonate, and frozen for 2h. Filter and collect the crystals, wash with water. The filtrate also contains the product which is further extracted with ethyl acetate. The obtained crystals can also be dissolved in the extract supplemented with ethyl acetate. Dry, filter, and concentrate to get 24 g product with yield 78%; Re-crystallize with isopropanol, m.p. 125~127 °C. |
| Chemical Properties | Crystalline Solid |
| Uses | Carbonic anhydrase inhibitor. Antiglaucoma agent |
| Uses | antihypertensive, beta-blocker, antianginal |
| Uses | For the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. |
| Brand name | Azopt (Alcon). |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
-
CAS:112529-15-4
$2.00 / 1kg
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $30.00/10mg |
VIP1Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $30.00/10mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $0.00/1g |
VIP1Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-09-05 | |
| $1.00/1g |
VIP1Y
|
Apeloa production Co.,Limited
|
2024-07-19 | |
| $0.00/1kg |
VIP2Y
|
Hangzhou ICH Biofarm Co., Ltd
|
2023-11-01 | |
| $35.00/1kg |
Shanghai Chinqesen Biotechnology Co., Ltd.
|
2023-10-30 | ||
| $35.00/1kg |
Hebei Xinsheng New Material Technology Co., LTD.
|
2023-10-16 | ||
| $50.00/1kg |
VIP5Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-09-13 | |
| $0.00/1g |
VIP2Y
|
shandong perfect biotechnology co.ltd
|
2023-08-03 | |
| $100.00/1g |
Shijiazhuang Gantuo Biotechnology Co., Ltd
|
2023-03-10 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com







 China
China