| Chemical Properties |
White to slightly yellow powder |
| Uses |
H1&2 agonist, edema induction, gastric secretion stimulant |
| Definition |
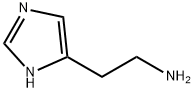
ChEBI: A member of the class of imidazoles that is 1H-imidazole substituted at position C-4 by a 2-aminoethyl group. |
| Indications |
Sinus problems, hay fever, bronchial asthma, hives, eczema, contact dermatitis, food allergies, and reactions to drugs are all allergic reactions associated with the release of histamine and other autocoids, such as serotonin, leukotrienes, and prostaglandins. Histamine release is frequently associated with various inflammatory states and may be increased in urticarial reactions, mastocytosis, and basophilia. Histamine also acts as a neurotransmitter in the central nervous system (CNS). Upon release from its storage sites, histamine exerts effects ranging from mild irritation and itching to anaphylactic shock and eventual death. |
| Biosynthesis |
Virtually all of the histamine found in individual organs and tissues is synthesized locally and stored in subcellular secretory granules. Within the tissues, the mast cells are the principal sites of storage; in the blood, the basophils serve this function. Histamine is also present in neurons of the CNS, where it acts as a neurotransmitter.
Histamine is synthesized from the amino acid histidine by an action of the enzyme histidine decarboxylase. Following synthesis, histamine is either rapidly inactivated or stored in the secretory granules of mast cells and basophils as an inactive complex with proteases and heparin sulfate or chondroitin sulfate. |
| Biological Functions |
Histamine occurs in the brain, particularly in certain hypothalamic neurons, and evidence is strong that histamine is a neurotransmitter. Distribution of histamine, its synthetic enzyme (histidine decarboxylase), and methyl histamine (the major brain metabolite) is not uniform. Possible roles for histamine in the regulation of food and water intake, thermoregulation, hormone release, and sleep have been suggested. |
| Mechanism of action |
Non–Antigen-Mediated Release of Histamine Histamine may be released from mast cells by mechanisms that do not require prior sensitization of the immune system. Drugs, high-molecular-weight proteins, venoms, and other substances that damage or disrupt cell membranes can induce the release of histamine. Any thermal or mechanical stress of sufficient intensity also will result in histamine release. Cytotoxic compounds, may release histamine as the result of disruption of cell membranes. |
| Pharmacology |
Histamine is found in animal tissues and venoms and in many bacteria and plants.Within the human body, the largest histamine concentrations are in the skin, lungs, and gastrointestinal mucosa, while concentrations are smaller in almost all other organs and tissues.Histamine is present in human plasma at relatively low concentrations (usually less than 0.5 ng/mL); in contrast, wholeblood levels can be as high as 30-fold greater. Substantial quantities of histamine are present in urine, with excretion rates varying from 10 to 40μg per 24 hours. |
| Clinical Use |
Histamine has only minor uses in clinical medicine. In the past it was used to diagnose pernicious anemia, in which histamine fails to evoke the usual secretion of gastric acid. Histamine has been used to assess bronchial hyperreactivity, although this test may be quite hazardous for asthmatics. Today the main clinical use of histamine is as a positive control injection for allergy skin testing. |
| Side effects |
Sedation is the most frequent adverse reaction to the first-generation antihistamines. An additive effect on alertness and motor skills will result if alcohol or another depressant is taken with these drugs. Antimuscarinic effects caused by these drugs include dry mouth and respiratory passages, urinary retention, and dysuria. Nausea, vomiting, constipation or diarrhea, dizziness, insomnia, nervousness, and fatigue also have been reported. Drug allergy, especially after topical application, is fairly common.Tolerance to certain antihistamines may develop after prolonged administration. Teratogenic effects of the piperazine antihistamines have been shown in animal studies. Epidemiological studies have not shown such an association in humans. The effects of toxic doses of first-generation antihistamines, similar to those seen following atropine administration, include excitement, hallucinations, dry mouth, dilated pupils, flushing, convulsions, urinary retention, sinus tachycardia, coma, and death.
The second-generation H1-antagonists are often referred to as nonsedating antihistamines; however, doses above the usual therapeutic level can cause sleepiness in certain individuals.A more serious adverse effect of some earlier second-generation antihistamines is cardiotoxicity. |
| Purification Methods |
It crystallises from *benzene or chloroform. [Beilstein 25 I 628, 25 II 302, 25 III/IV 2049.] |






 China
China