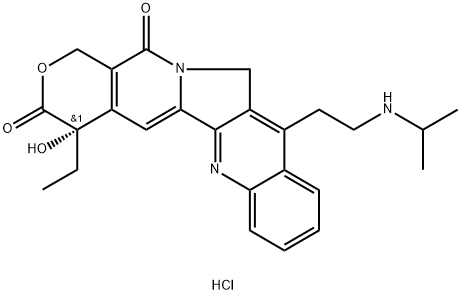
Camtobell hydrochloride
| Price | $3 |
| Package | 3ASSAYS |
| Min. Order: | 1KG |
| Supply Ability: | 100kg |
| Update Time: | 2019-07-10 |
Product Details
| Product Name: Camtobell hydrochloride | CAS No.: 213819-48-8 |
| Min. Order: 1KG | Purity: 99% |
| Supply Ability: 100kg | Release date: 2019/07/10 |
| Product No.: windy |
| Camtobell hydrochloride Basic information |
| Product Name: | Camtobell hydrochloride |
| Synonyms: | Belotecan hydrochloride(CKD-602);BELOTECAN HYDROCHLORIDE;CKD-602: BELOTECAN HYDROCHLORIDE;(S)-4-Ethyl-4-hydroxy-11-[2-(isopropylamino)ethyl]-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione hydrochloride;Camtobell hydrochloride;(S)-4-Ethyl-4-hydroxy-11-(2-(isopropylamino)ethyl)-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinol;Belotecan HCl |
| CAS: | 213819-48-8 |
| MF: | C25H27N3O4.HCl |
| MW: | 469.97 |
| EINECS: | |
| Product Categories: | Amines;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Amines, Chiral Reagents, Pharmaceuticals, Intermediates & Fine Chemicals |
| Mol File: | 213819-48-8.mol |
 |
|
| Camtobell hydrochloride Chemical Properties |
| Safety Information |
| MSDS Information |
| Camtobell hydrochloride Usage And Synthesis |
| Description | Camtobell hydrochloride, a DNA topoisomerase I inhibitor, is an analog of camptothecin. It was launched in the Republic of Korea as an injectable formulation for the treatment of ovarian and small cell lung cancer. Although camptothecin exhibits potent antineoplastic activity in vitro, its clinical application is hampered by severe toxicity and poor water solubility. Several synthetic and semi-synthetic analogs of camptothecin with improved solubility and lower toxicity have been developed over the past two decades. Two drugs from this class, topotecan and irinotecan, have been launched in previous years and belotecan is the newest member to reach the market. Camtobell hydrochloride is prepared by a two-step semi-synthesis starting from camptothecin, first by converting to 7-methylcamptothecin via a free-radical methylation reaction using a combination of acetic acid, tert-butylhydroperoxide, ferrous sulfate and sulfuric acid, and subsequently, in the second step, a Mannich reaction with isopropylamine hydrochloride and dimethylsulfoxide. A total synthesis of belotecan in seventeen steps starting from ethyl acetopyruvate is also reported. Belotecan inhibits topoisomerase I with approximately equal potency as camptothecin and about 3-fold higher potency than topotecan, with respective IC50 values of 0.119, 0.123 and 0.33 μg/mL. Its cytotoxic activity is comparable to that of camptothecin, with IC50 values ranging from 2 ng/mL to 2 μg/mL against 26 different human cancer cell lines. In studies using human tumor xenografts in nude mice, 80-100 mg/ kg of belotecan dosed every four days for four doses produced 67 to 94% tumor regression rates against HT-29, WIDR and CX-1 colon, LX-1 lung, MX-1 breast and SKOV-3 ovarian carcinomas. Pharmacokinetic studies of camtobell hydrochloride in rats at intravenous doses of 2.6–8.9 mg/kg demonstrated that both Cmax and AUC increased in a dose-dependent manner. Total clearances, volumes of distribution and mean residence times did not change significantly with increasing doses. The elimination half-life ranged between 9.2 to 11.2 hours. In a Phase I study of camtobell hydrochloride, the fraction of renal clearance was found to be 33.1 to 50.3%, and the protein-binding fraction was 53 to 87%. Approximately 9.5% of the administered dose was excreted via the hepatobiliary system. In clinical studies involving 20 patients with recurrent or refractory ovarian cancer, intravenous administration of 0.5 mg/m2/day of belotecan for 5 days every 3 weeks over a median of six dosing cycles resulted in an overall response rate of 45%. All patients had grade 3 or 4 neutropenia as the most significant adverse event. |
| Originator | CKD Pharmaceuticals (S. Korea) |
| Uses | A novel camptothecin-derivative anti-tumor agent. CKD-602-related toxicities induced by IV infusion administration have not yet been evaluated, although the drug is more widely used in clinical settings. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1KG |
VIP2Y
|
Henan Aochuang Chemical Co.,Ltd.
|
2022-10-13 | |
| $10.00/1kg |
VIP1Y
|
Hunan aslsen technology co.,ltd
|
2024-07-04 | |
| $2900.00/1g |
VIP1Y
|
R&D Scientific Inc.
|
2024-05-02 | |
| $0.00/10mg |
VIP1Y
|
Shenzhen Hengfeng Wanda Pharmaceutical Technology Co., Ltd
|
2024-04-30 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com




 China
China