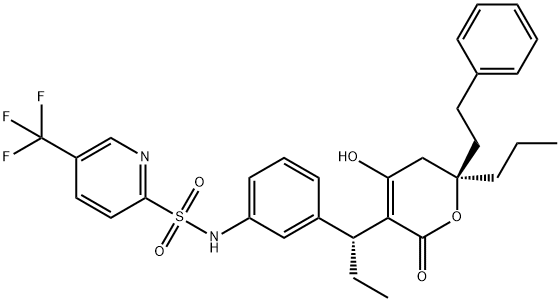
Tipranavir
| Price | $3 |
| Package | 3KG |
| Min. Order: | 1KG |
| Supply Ability: | 100kg |
| Update Time: | 2019-07-12 |
Product Details
| Product Name: Tipranavir | CAS No.: 174484-41-4 |
| Min. Order: 1KG | Purity: 99% |
| Supply Ability: 100kg | Release date: 2019/07/12 |
| Contact: windy | Email: windy@coreychem.com |
| Tel: +86 13017688754 |
| Tipranavir Basic information |
| Product Name: | Tipranavir |
| Synonyms: | PNU-140690;Aptivus;N-[3-[(1R)-1-[(6R)-5,6-Dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2H-pyran-3-yl]propyl]phenyl]-5-(trifluoromethyl)-2-pyridinesulfonamide;N-[3-[(1R)-1-[(6R)-2-Hydroxy-4-oxo-6-phenethyl-6-propyl-5H-pyran-3-yl]propyl]phenyl]-5-(trifluoromethyl)pyridine-2-sulfonamide;Tipranavir;Tipranavir(PNU-140690,Aptivus);2-PyridinesulfonaMide,N-[3-[(1R)-1-[(6R)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2H-pyran-3-yl]propyl]phenyl]-5-(trifluoroMethyl)- |
| CAS: | 174484-41-4 |
| MF: | C31H33F3N2O5S |
| MW: | 602.66 |
| EINECS: | |
| Product Categories: | Antiviral Agents;All Inhibitors;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals |
| Mol File: | 174484-41-4.mol |
 |
|
| Tipranavir Chemical Properties |
| Melting point | 86-890C |
| alpha | D +20° (ethanol) |
| storage temp. | -20°C Freezer |
| Safety Information |
| MSDS Information |
| Tipranavir Usage And Synthesis |
| Description | Tipranavir, an oral non-peptidic HIV protease inhibitor, was granted accelerated approval for use in combination with ritanovir and was subsequently launched the same month in 2005. The targeted patient population includes HIV-1 infected adults with evidence of viral replication and demonstrated resistance to multiple protease inhibitors. As with other protease inhibitors, binding at the HIV-1 protease’s active site inhibits the virus-specific processing of the Gag and Gag-Pol polyproteins in HIV-1-infected cells resulting in the production of non-infectious virions. An effective regimen to reduce viral load and to preserve immune function typically consists of a cocktail of a protease inhibitor and at least one nucleoside reverse transcriptase inhibitor. It is believed that tipranavir has been effective where other protease inhibitors have encountered resistance because, as a non-peptidic inhibitor, it was designed by structure-based analysis to have increased flexibility making it acquiescent to conformational alterations at the binding site. Boosting with ritonavir (200 mg) increased tipranavir Cmin 4-fold and Cmax 20-fold. The primary route of excretion is through the feces (83%) with an effective mean elimination half-life of 4.8 h in healthy volunteers and 6.0 h in HIV-infected adults. The efficacy of tipranavir/ritonavir combination therapy has been evaluated in multiple clinical trials. In general, the adverse events included nausea, vomiting, and diarrhea. While tipranavir is a CYP3A4 inducer and substrate, as previously stated, its co-administration with the CYP3A4 inhibitor ritonavir results in net inhibition; therefore, patients should avoid the concomitant use of drugs highly dependent on CYP3A4 for clearance. The complete list of contraindicated drugs can be found in the package insert, but the general classes include antiarrhythmics, antihistamines, antimycobacterials, neuroleptics, sedatives, ergot derivatives, GI motility agents, and the herbal supplement St. John’s wort. Finally, as the elevated liver enzyme levels suggest, tipranavir should not be taken by patients with severe liver disease. Patients with clinical symptoms of hepatitis should immediately discontinue use of tipranavir. It is highly recommended that liver function tests be performed prior to treatment and throughout the course of therapy. |
| Chemical Properties | White Solid |
| Originator | Pharmacia &Upjohn (US) |
| Uses | Nonpeptidic HIV protease inhibitor (NPPI). Antiviral |
| Definition | ChEBI: A pyridine-2-sulfonamide substituted at C-5 by a trifluoromethyl group and at the sulfonamide nitrogen by a dihydropyrone-containing m-tolyl substituent. It is an HIV-1 protease inhibitor. |
| Brand name | Aptivus |
| Acquired resistance | In a study of 105 viruses resistant to other protease inhibitors, 90% exhibited a more than four-fold decrease in susceptibility and 2% high-level resistance (>10-fold decrease). The predominant emerging mutations in use with ritonavir are L33F/I/V, V82T/L and I84V. Combination of all three of these mutations is usually required for reduced susceptibility. Mutations at positions 47, 58 and 74 are also associated with resistance. |
| General Description | Tipranavir is unique among the PIs because it is not a peptidomimeticcompound. It does appear to bind to the activesite of HIV-1 protease the same as the peptidomimetics do.The benefit of this agent is that, because it is a differentchemical structure, cross-resistance does not develop tothe same extent as seen with the peptidomimetics. Thedrug is administered with a booster dose of ritonavir. Thisprotocol inhibits CYP3A4, causing the levels of tipranavirto increase. |
| Pharmaceutical Applications | A non-peptidic protease inhibitor formulated as capsules or solution for oral use. |
| Pharmacokinetics | Oral absorption: Not known/available Cmax 500 mg + 200 mg ritonavir twice: c. 57.2 mg/L (female); daily: 46.8 mg/L (male) Cmin 500 mg + 200 mg ritonavir twice: c. 25.1 mg/L (female); daily: 21.5 mg/L (male) Plasma half-life: c. 5.5 h (female); 6 h (male) Volume of distribution: Not known/available Plasma protein binding: >99.9% Absorption and distribution The combination with ritonavir may be taken with or without food. No studies have been conducted to determine the distribution into human CSF, semen or breast milk. Metabolism and excretion Metabolism in the presence of 200 mg ritonavir is minimal. Around 82% is excreted in the feces and 4% in the urine. In mild hepatic impairment it should be used with caution; it should not be used in moderate or severe hepatic impairment. |
| Clinical Use | Treatment (in combination with other antiretroviral drugs) of HIV-1 infection in patients unresponsive to more than one other protease inhibitor |
| Side effects | Adverse effects include nausea, vomiting, diarrhea, fatigue and headache. In studies of ritonavir-boosted regimens higher rates of hepatotoxicity have been observed with tipranavir than with other protease inhibitors. In addition, 14 reports of intracranial bleeding (eight fatal cases) associated with tipranavir have been reported. It has been associated with dyslipidemia to a greater extent than other protease inhibitors. |
| Tipranavir Preparation Products And Raw materials |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-09 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com




 China
China