
Product Details
| Product Name: Azilsartan impurity Q | CAS No.: 1604812-35-2 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 1000000000000 | Release date: 2025/02/22 |

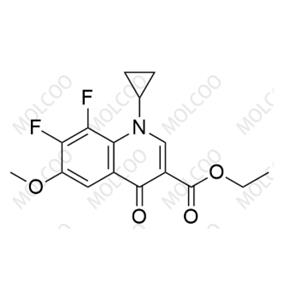
CAS:1604812-35-2
We can provide a full range of impurity reference/standard products required for drug development. Most of the impurities are synthesized through the process, there are also many items of impurities can not be obtained by synthetic means, need to be obtained through raw materials, intermediates, crude products or side reactions contained in the trace target compounds, Hubei Moke has a professional impurity preparation and separation technology team, equipped with professional SFC preparation and separation equipment. It can carry out efficient and accurate separation of impurities for complex projects, and solve the problem of impurity preparation for customers.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-06-03 | |
| $0.00/10mg |
Hangzhou Huarong Pharm Co., Ltd.
|
2021-09-17 | ||
| $1.00/1g |
VIP6Y
|
Career Henan Chemical Co
|
2019-12-30 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake






 China
China