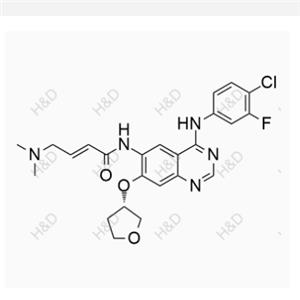
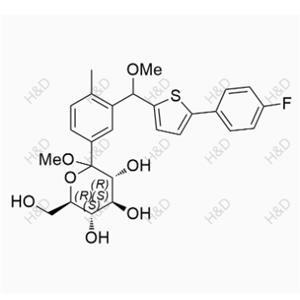
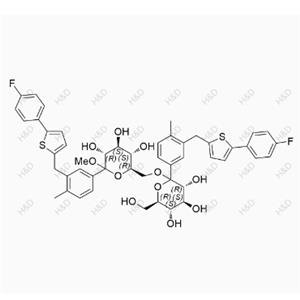
Canagliflozin Hydroperoxide Impurity
| Price | Get Latest Price |
| Package | 10mg |
| Min. Order: | 10mg |
| Supply Ability: | 1g |
| Update Time: | 2024-08-28 |
Product Details
| Product Name: Canagliflozin Hydroperoxide Impurity | CAS No.: 2247196-28-5 |
| Min. Order: 10mg | Purity: 95% |
| Supply Ability: 1g | Release date: 2024/08/28 |
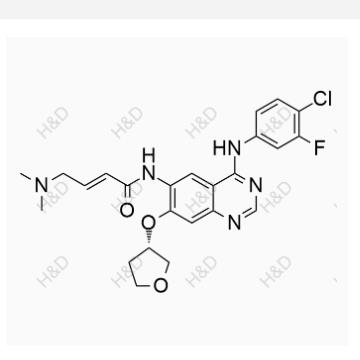
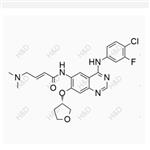
Chinese name :Caglil net peroxide impurity
English name :Canagliflozin Hydroperoxide Impurity
CAS number 2247196-28-5
Molecular form: C24H25FO7S
Molecular weight :476.51
The company focuses on providing pharmaceutical research and development units with new drug and generic drug registration and application of chemical products, including drug standards, drug impurity reference products, chemical reagents, the one-time import of original reference preparations, joint laboratory development of drugs and intermediates process development and production, compound custom synthesis services, as well as drug impurity preparation separation and purification, unknown impurity preparation separation and structure Appraise.
Can provide COA quality report, hydrogen spectrum, mass spectrometry, liquid phase (HPLC), can also provide carbon spectrum, ultraviolet, infrared, two-dimensional spectrum.
The default delivery standard of our H&D products is: the purity of liquid phase (HPLC) is not less than 95%, and if the impurity is less than 95%, which is very difficult to purify, we will tell the customer specially when quoting. The project has a complete set of impurities, off-the-shelf supply,1-2 weeks of goods.
Add my contact information and provide the impurity chemical structure formula and CAS number of many items. These impurity structural formulas are very helpful to your R&D personnel. The R&D personnel can see the popular impurity structural formulas of many projects on our website, which can reduce the time for your R&D personnel to speculate the chemical structural formulas. New and old customers we can also provide spectrum analysis services, provide synthetic routes. From stock, 1 to 2 weeks.
Impurity Sales Manager: Manager Sun
Impurity consultation phone: +86-13627253706
Impurity enterprise QQ: 2881497699
email: sale@hdimpurity.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/25kg |
VIP1Y
|
PUSHAN INDUSTRIAL (SHAANXI) CO.,LTD
|
2024-05-17 | |
| $0.00/25Kg/Bag |
VIP4Y
|
Sinoway Industrial co., ltd.
|
2023-05-29 | |
| $5.00/1kg |
VIP3Y
|
Hebei Fengjia New Material Co., Ltd
|
2024-05-29 |



 China
China