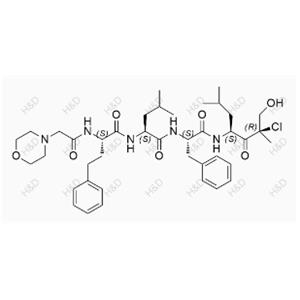
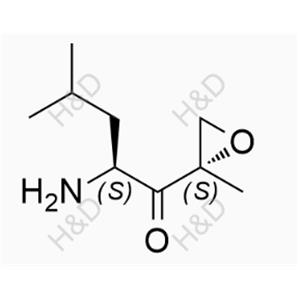
Carfilzomib Impurity 57 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 25mg | 50mg |
| Min. Order: | 10mg |
| Supply Ability: | 500mg |
| Update Time: | 2024-09-20 |
Product Details
| Product Name: Carfilzomib Impurity 57 | Min. Order: 10mg |
| Purity: 98% | Supply Ability: 500mg |
| Release date: 2024/09/20 |

CAS Number:
N/A
Molecular formula:
C40H58ClN5O7
Molecular weight:
756.37
Email:sale@hdimpurity.com
Price wire negotiation
ShenZhen H&D Pharmaceutical Technology Co., LTD is a professional supplier of drug impurity control products. Products cove more than 90% of cutting-edge generic drug projects. All products must check LCMS and HPLC within a year.We provide customized synthesis and preparation of small molecule compounds.
ShenZhen H&D Pharmaceutical Technology Co., LTD has its own laboratory. The company focuses on providing pharmaceutical research and development units with new drug and generic drug registration and application of chemical products.

The company focuses on providing pharmaceutical research and development units with new drugs and generic drug registration and application of chemical products, including drug impurity reference products and standard products. Our delivery standard is not less than 98% purity (HPLC).If the purity is less than 98%, we will give special instructions to the customer.
Impurity Sales Manager: Mr. Sun
Email:sale@hdimpurity.com
Price wire negotiation
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/25Kg/Bag |
VIP4Y
|
Sinoway Industrial co., ltd.
|
2023-05-11 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2024-12-31 | |
| $0.00/1KG |
VIP1Y
|
Hangzhou Hyper Chemicals Limited
|
2024-05-09 |



 China
China