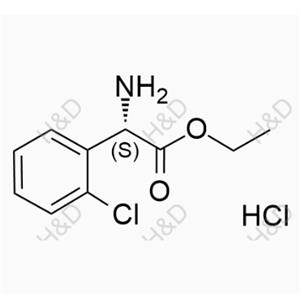
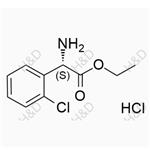
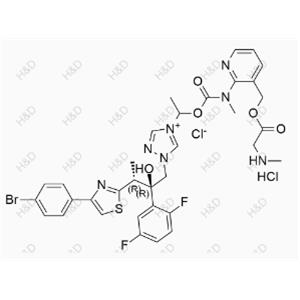
Clopidogrel Impurity 47 (Hydrochloride) NEW
| Price | Get Latest Price | ||
| Package | 10mg | 25mg | 50mg |
| Min. Order: | 10mg |
| Supply Ability: | 10g |
| Update Time: | 2025-03-04 |
Product Details
| Product Name: Clopidogrel Impurity 47 (Hydrochloride) | Min. Order: 10mg |
| Purity: 0.98 | Supply Ability: 10g |
| Release date: 2025/03/04 |
CAS Number:
N/A
Molecular formula:
C10H12ClNO2.HCl
Molecular weight:
213.06 35.98
The company focuses on providing pharmaceutical research and development units with new drugs and generic drug registration and application of chemical products, including drug impurity reference products, standard products, pharmaceutical intermediates, and drug impurity preparation, separation and purification business. Our company is located in Wuhan, a city with abundant talents and a thoroughfare of nine provinces.The company enjoys more rich academic foundation and convenient and fast logistics speed.
Our delivery standard is not less than 98% purity (HPLC).If the purity is less than 98%, we will give special instructions to the customer. All standard products will be provided by Molcoo quality assurance and related certificates (COA), H-NMR, MS, HPLC and other structure confirmation to meet the basic needs.Customers can also test C-NMR, QNMR, IR, etc., according to their own needs, we can also provide atlas analysis services for new and old customers Provides a simple synthetic route.
We can provide ull set of stock.Our products are subject to strict quality control to ensure excellent quality. So we will carry out strict quality testing on each product before delivery. We also have a professional sales customer service team , so that you can rest assured before and after sales.
Impurity Sales Manager: Manager Sun
WhatsApp account:+86 136 2725 3706
Email:sale@hdimpurity.com
Price wire negotiation
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-06 | |
| $0.00/10mg |
VIP1Y
|
Moxin Chemicals
|
2025-01-15 | |
| $990.00/1ton |
VIP1Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-19 |



 China
China