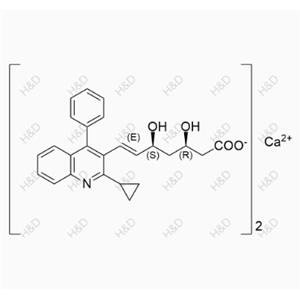
Pitavastatin Impurity 54
| Price | Get Latest Price | |
| Package | 5mg | 10mg |
| Min. Order: | 10mg |
| Supply Ability: | 10g |
| Update Time: | 2025-03-04 |
Product Details
| Product Name: Pitavastatin Impurity 54 | CAS No.: 1258947-30-6 |
| Min. Order: 10mg | Purity: 0.98 |
| Supply Ability: 10g | Release date: 2025/03/04 |
The company has its own research and development center, focusing on providing pharmaceutical research and development units with new drug and generic drug registration and application of chemical products, including drug standards, drug impurity reference products, characteristic intermediates, analysis and testing and customized compound synthesis services. The company can provide professional technical services.
The company focuses on providing pharmaceutical research and development units with new drugs and generic drug registration and application of chemical products, including drug impurity reference products, standard products, pharmaceutical intermediates, and drug impurity preparation, separation and purification business. Our company is located in Wuhan, a city with abundant talents and a thoroughfare of nine provinces.The company enjoys more rich academic foundation and convenient and fast logistics speed.
Our delivery standard is not less than 98% purity (HPLC).If the purity is less than 98%, we will give special instructions to the customer. All standard products will be provided by Molcoo quality assurance and related certificates (COA), H-NMR, MS, HPLC and other structure confirmation to meet the basic needs.Customers can also test C-NMR, QNMR, IR, etc., according to their own needs, we can also provide atlas analysis services for new and old customers Provides a simple synthetic route.
Impurity Sales Manager: Manager Sun
Impurity consultation phone: +8613627253706
Email:sale@hdimpurity.com
WhatsApp:ocean
Price wire negotiation
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
Moxin Chemicals
|
2025-01-16 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-02-26 | |
| $0.00/25Kg/Bag |
VIP4Y
|
Sinoway Industrial co., ltd.
|
2022-08-09 |
- Since: 2014-09-01
- Address: Biwangxin Technology Building 503, Biwangxin High-tech Industrial Park, Xili Street, Nanshan Distric




 China
China