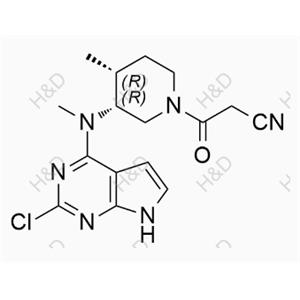
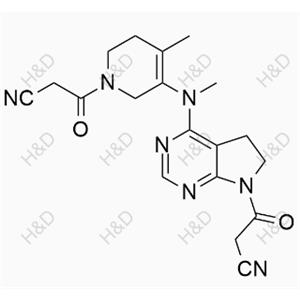
Tofacitinib Impurity G
| Price | Get Latest Price | ||
| Package | 10mg | 25mg | 50mg |
| Min. Order: | 10mg |
| Supply Ability: | 10g |
| Update Time: | 2025-03-04 |
Product Details
| Product Name: Tofacitinib Impurity G | CAS No.: 1616761-00-2 |
| Min. Order: 10mg | Purity: 0.98 |
| Supply Ability: 10g | Release date: 2025/03/04 |
CAS Number:
1616761-00-2
Molecular formula:
C16H19ClN6O
Molecular weight:
346.81
Email:sale@hdimpurity.com
Price wire negotiation
Shenzhen Hengfeng Wanda Pharmaceutical Technology is a professional supplier of drug impurity control products. Products cove more than 90% of cutting-edge generic drug projects. All products must check LCMS and HPLC within a year.We provide customized synthesis and preparation of small molecule compounds.
The company has its own research and development center, focusing on providing pharmaceutical research and development units with new drug and generic drug registration and application of chemical products, including drug standards, drug impurity reference products, characteristic intermediates, analysis and testing and customized compound synthesis services.
The company focuses on providing pharmaceutical research and development units with new drugs and generic drug registration and application of chemical products, including drug impurity reference products, standard products, pharmaceutical intermediates, and drug impurity preparation, separation and purification business. Our delivery standard is not less than 98% purity (HPLC).If the purity is less than 98%, we will give special instructions to the customer.
Impurity Sales Manager: Mr. Sun
Email:sale@hdimpurity.com
Price wire negotiation
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
Moxin Chemicals
|
2025-04-02 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-24 | |
| $0.00/10g |
VIP1Y
|
Cangzhou Kangrui Pharma Tech Co. Ltd.,
|
2024-11-04 | |
| $0.00/100mg |
VIP1Y
|
Moxin Chemicals
|
2025-02-12 | |
| $1.00/1kg |
VIP7Y
|
Career Henan Chemical Co
|
2018-12-24 |



 China
China