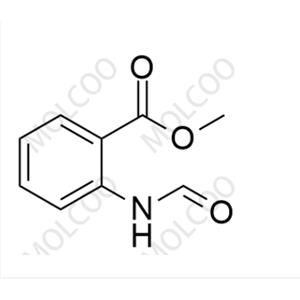
Tranilast Impurity 14 NEW
| Price | Get Latest Price | ||
| Package | 30mg | 50mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-04-22 |
Product Details
| Product Name: Tranilast Impurity 14 | CAS No.: 41270-80-8 |
| Min. Order: 10mg | Purity: 98 |
| Supply Ability: 100000 | Release date: 2025/04/22 |
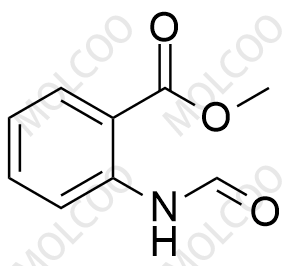
I. Product Name
Tranilast Impurity Reference Standard
II. Product Description
The Tranilast Impurity Reference Standard is a crucial substance used in drug quality control and research and development processes, specifically for detecting and identifying potential impurities in Tranilast drugs. These impurities may originate from various factors such as raw materials, production processes, or storage conditions. By comparing with the impurity reference standard, the types and quantities of impurities in Tranilast drugs can be accurately determined, ensuring the safety and effectiveness of the drugs.
III. Product Characteristics
High Purity: The impurity reference standard itself has high purity to ensure the accuracy of test results.
Structural Stability: Under specified storage conditions, the impurity reference standard remains stable and is not prone to structural changes.
Wide Applicability: It is suitable for multiple analytical techniques, such as High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC), meeting different testing needs.
IV. Application Fields
The Tranilast Impurity Reference Standard is widely used in drug research and development, production, quality control, and drug regulation fields. It is an important tool for ensuring the quality and safety of Tranilast drugs.
V. Storage Conditions
It should be stored in a dry, cool, and light-protected environment, avoiding high temperatures, humidity, and direct sunlight.
VI. Precautions
Before use, please read the product instruction manual carefully to understand the product characteristics and usage methods.
Avoid contamination or cross-contamination of the impurity reference standard.
Use within the validity period; do not use expired products.
During storage and use, strictly comply with relevant safety regulations and operating procedures.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1kg |
VIP1Y
|
Xi an Biohorlden Industry Trade Co Ltd
|
2025-04-11 | |
| $1.00/1g |
VIP4Y
|
WUHAN FORTUNA CHEMICAL CO., LTD
|
2021-06-21 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-25 | |
| $0.00/1kg |
VIP1Y
|
JINING XINHE CHEMICAL CO., LTD
|
2024-07-20 |






 China
China