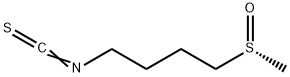
(R)-sulforaphane- Pharmacologic Action
Dec 17,2019
Sulforaphane (SFN) is an anti-cancer, anti-microbial and anti-diabetic compound found in cruciferous vegetables [1,2]. It induces the production of detoxifying enzymes such as quinone reductase and glutathione S-transferase that cause xenobiotic transformation. Sulforaphane also increases the transcription of tumor suppressor proteins and inhibits histone deacetylases. It modulates inflammatory responses by suppressing the LPS-mediated expression of iNOS, COX-2, IL-1β and TNF-α[3]. SFN also has a marked effect on cell cycle checkpoint controls and cell survival and/or apoptosis in various cancer cells, through mechanisms that are poorly understood.
Sulforaphane (SFN) acts as an inhibitor of histone deacetylase (HDAC). In human embryonic kidney 293 cells, SFN dose-dependently increased the activity of a β-catenin-responsive reporter (TOPflash), without altering β-catenin or HDAC protein levels. Cytoplasmic and nuclear extracts from these cells had diminished HDAC activity, and both global and localized histone acetylation was increased, compared with untreated controls. Studies with Sulforaphane (SFN) and with media from SFN-treated cells indicated that the parent compound was not responsible for the inhibition of HDAC, and this was confirmed using an inhibitor of glutathione S-transferase, which blocked the first step in the metabolism of SFN, via the mercapturic acid pathway. Whereas Sulforaphane (SFN) and its glutathione conjugate (SFN-GSH) had little or no effect, the two major metabolites SFN-cysteine and SFN-N-acetylcysteine were effective HDAC inhibitors in vitro. SFN dose-dependently increased TOPflash reporter activity and inhibited HDAC activity, there was an increase in acetylated histones and in p21Cip1/Waf1, and chromatin immunoprecipitation assays revealed an increase in acetylated histones bound to the P21 promoter.
Sulforaphane (SFN) may be effective as a tumor-suppressing agent and as a chemotherapeutic agent, alone or in combination with other HDAC inhibitors currently undergoing clinical trials[4]. The chemopreventive activity of the phytochemical sulforaphane, (‐)1‐isothiocyanato‐4R‐(methylsulfinyl)‐butane, present in cruciferous vegetables in substantial amounts in the form of glucosinolate, was demonstrated in animal models of cancer using the racemate, despite the fact that humans are exposed only to the R‐enantiomer through the diet. Since a principal mechanism of the chemopreventive activity of sulforaphane is modulation of the carcinogen‐metabolising enzyme systems, a study was conducted in precision‐cut rat liver and lung slices, and in FAO cells comparing the ability of R‐ and S‐sulforaphane to modulate these enzyme systems.
R‐sulforaphane elevated hepatic glutathione S‐transferase and quinone reductase whereas the S‐enantiomer had no effect; moreover, the R‐enantiomer was more effective in up‐regulating GSTα, GSTμ and quinone reductase protein levels. In the lung, both enantiomers increased the same enzyme activities with the R‐enantiomer being more potent; in addition, the R‐enantiomer was more effective in up‐regulating GSTα and quinone reductase protein levels. Both isomers increased glutathione levels in both tissues, with R‐sulforaphane being more potent. Finally, R‐sulforaphane was the more effective of the two isomers in up‐regulating CYP1A1/1B1 apoprotein levels in both liver and lung, and CYP1A2 in the liver. Similarly, in FAO cells the R‐enantiomer was far more effective in up‐regulating quinone reductase and glutathione S‐transferase activities and protein levels compared with the S‐isomer. These studies demonstrate clearly the superiority of R‐sulforaphane, when compared with the S‐enantiomer, in stimulating detoxification enzymes, and raises the possibility that the animal studies that employed the racemate may have underestimated the chemopreventive activity of this isothiocyanate[5].
References
1.David O, et. al. Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming[J]. Nature communications, 2018, 9(1):3506.
2.Eugene YHY, et. al. Identification of Ginkgo biloba as a novel activator of pregnane X receptor[J]. Drug metabolism and disposition: the biological fate of chemicals, 2008, 36(11): 2270
3.Samuele M. et. al. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter[J]. Haematologica, 2010, 95(8):1261-1268.
4.Melinda C, Myzak, P. Andrew K, Fung-Lung C, and Roderick H. A Novel Mechanism of Chemoprotection by Sulforaphane. Cancer Research, 2004, 64:5767–5774
5.Abdull Razis AF, Iori R, Ioannides C. The natural chemopreventive phytochemical R‐sulforaphane is a far more potent inducer of the carcinogen‐detoxifying enzyme systems in rat liver and lung than the S‐isomer. International Journal of Cancer. 2011, 128(12):2775-8
- Related articles
- Related Qustion
- L-Sulforaphane: mechanism of action and clinical applications Nov 30, 2023
L-Sulforaphane from cruciferous vegetables activates antioxidant and anti-inflammatory pathways, showing potential for medical applications.
2-Hydroxy-2-methylpropiophenone is a photoinitiator. In addition, 2-hydroxy-2-methylpropiophenone is an important organic intermediate (building block) to synthetize substituted propiophenone products.....
Dec 17,2019Catalyst and Auxiliary2-Amino-2-methyl-1-propanol is an important organic intermediate (building block) to synthetize substituted amino products.....
Dec 17,2019Organic reagentsL-Sulforaphane
142825-10-3You may like
- Synephrine: use,mechanism and risk assessment
Nov 3, 2025
- Pharmacology research of Genipin
Oct 15, 2025
- What are the Benefits of Malt Extract for the Humans?
Jul 31, 2025
- (R)-Sulforaphane
-

- $57.00 / 1mg
- 2025-11-05
- CAS:142825-10-3
- Min. Order:
- Purity: 98.73%
- Supply Ability: 10g
- L-Sulforaphane
-

- $0.00 / 1KG
- 2025-11-05
- CAS:142825-10-3
- Min. Order: 1KG
- Purity: 0.6%-98% HP0LC
- Supply Ability: 1000KG
- Sulforaphane
-

- $0.00 / 25kg
- 2025-06-13
- CAS:142825-10-3
- Min. Order: 1kg
- Purity: 1%-10% 98%
- Supply Ability: 1000 kg