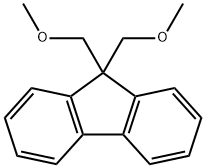
9, 9-Bis (methoxymethyl) fluorene: Synthesis and Application
Dec 7,2022
General description
The 9,9-Bis(methoxymethyl) fluorene proved to be one of the best electron donors with heterogeneous Ziegler-Natta catalysts. The unique electronic effect and steric effect can make the active center of catalyst have good selectivity and high stereoorientation. Using 9,9-Bis(methoxymethyl) fluorene as the internal electron donor of Ziegler-Natta catalyst, polypropylene with relatively narrow molecular mass distribution, high isometric and good mechanical properties could be prepared. In addition, the compound can also be used as an intermediate, through polymerization reaction or functional group, applied to materials or drug molecules and other domains. Therefore, the compound has a wide range of applications.
Synthetic routes
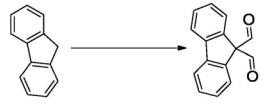
Fig. 1 The synthetic step 1 of 9,9-Bis(methoxymethyl) fluorene.
Take 500 mL three-nozzle flask, add potassium ethanol (45.4 g, 0.54 mol) and ether (150 mL) under nitrogen protection, transfer the flask to the ice water bath; Then the diethyl ether (100 mL) solution of fluorene (15 g, 0.09 mol) was added. After the drip was completed, the reaction was continued for 1 hour, and then ethyl formate (40 g, 0.54 mol) was added. Finally, the temperature is slowly raised to room temperature, and the reaction continues until TLC shows complete transformation of the raw material. Then, water quenching reaction was added, ethyl acetate extraction was used, organic phase was washed with saturated salt water, dried, and solvent was steamed to get crude product, which was not purified and directly used for the next reaction [1].
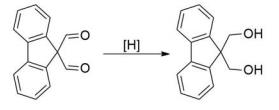
Fig. 2 The synthetic step 2 of 9,9-Bis(methoxymethyl) fluorene.
Take 500 mL three-way flask and add the crude product of the previous step and 200 mL methanol, stir to dissolve, and then add sodium boron ammoniate (6.8 g, 0.18 mol) in batches, reaction at room temperature until TLC shows the complete transformation of raw materials. Then, the filtrate was filtered, the filtrate was collected, the solvent was dried, and the remaining solid was purified by toluene recrystallization to get 18.3 g 9, 9-fluorene dimethyl alcohol (the total yield of the two-step reaction is 90%, and the purity of 9, 9-fluorene dimethyl alcohol is 98%) [2].
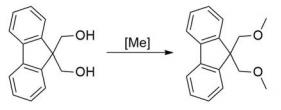
Fig. 3 The synthetic step 3 of 9,9-Bis(methoxymethyl) fluorene.
The 100 mL tube sealed reaction bottle with magneton was dried and added with 9, 9-fluorene dimethyl alcohol (8.5 g, 0.04 mol), trimethyl phosphonate (14 mL,0.12 mol) and ferrous trifluoromesylate (0.56 g,1.6 mol). The bottle was sealed and heated to 100℃ for reaction for 24 hours. After the reaction, it was cooled to room temperature, methanol was added to the reaction solution, and the target product was precipitated and filtered to obtain the target product. After atmospheric distillation and vacuum distillation, methanol and trimethyl phosphate were recovered and recrystallized with methanol, part of the products could be obtained again. Finally, the target product 9, 9-bis (methoxymethyl) fluorene 9.3g was obtained, and the reaction yield was 92% (after gas chromatography detection, the purity of 9, 9-bis (methoxymethyl) fluorene was 98%) [1].
Application
As an Lewis base
In this work Credendino et al. present a systematic DFT analysis of the effect of surface coverage on the coordination properties of two industrial Lewis bases, dimethyl phthalate and 9,9-bis(methoxymethyl)fluorene, to the (104) and (110) surfaces of MgCl2. Further, we investigated several possible migration pathways for the migration of the Lewis bases on the same MgCl2 monolayer. Our study clearly shows that complete coverage of the Mg vacancies on the surface by coordinating dimethyl phthalate or 9,9-bis(methoxymethyl)fluorene is hampered by steric repulsion between vicinally coordinated donor molecules. Further, our study clearly indicates that both dimethyl phthalate and 9,9-bis(methoxymethyl)fluorene migration on the same MgCl2 monolayer on the (104) and (110) surfaces basically requires donor dissociation [2].
Infrared vibrational spectroscopy has been used to reveal the conformations of the Ziegler-Natta catalysts internal electron donor 9,9-bis(methoxymethyl)-fluorene and its selectively deuterated derivative (9,9-bis(1,1-dideutero-methoxymethyl)-fluorene), in the solid state, complexed with TiCl(4) and MgCl(2) and in the catalyst precursor. The experimental spectra have been interpreted by means of spectroscopic correlations and theoretical results from quantum chemical calculations on selected molecular models. From experimental and theoretical data two chelating geometries of the donors with both oxygen directly interacting with Ti or Mg atoms turn out to be favorable. They can be identified by the four dihedral angles along the CH(3)-(O-CH(2)-R-CH(2)-O)-CH(3) residue that take on conformations of approximately trans (T), gauche(+) (G), or gauche(-) (G'), namely, TOOT and TGG'T. We propose the TOOT conformations for the crystal phase of the donor, the TGG'T when it is complexed with TiCl(4), and both TGGT and TGG'T when complexed with MgCl(2) along the [110] lateral cut. In the precatalysts, donors molecules are complexed along the [110] MgCl(2) lateral cut and likewise the MgCl(2) complex. A model for the catalytic sites consisting of TiCl(4) species and donor molecules complexed along the [110] lateral cut is proposed [3].
As a catalyst
All polymerization experiments were carried out in a Buchi glass reactor capable of operating at propylene pressures of 1-7 atmospheres. Two types of MgCl2 supported catalysts with controlled morphology were studied, viz., catalyst systems containing either a 1,3 diether (Catalysts A and D), 9,9-bis (methoxymethyl) fluorene, or a simple diester (Catalysts B, C and E) such as diisobutylphthalate, used as reference systems. The kinetic behaviours of these catalysts were investigated for propylene polymerization. The 1,3-diether based systems proved to be excellent catalyst systems for propylene polymerization showing high polymerization activities and stable rate-time profiles and produced spherical polymer particles. Catalyst pre-treatment was found to play an important role in controlling the morphology of the final polymer. A tritiated alcohol radio-quenching technique was used to determine the concentrations active centres: values of 7.5 and 5.1 mol/mol % respectively were obtained for catalysts A and D and 3.5, 2.9 and 2.2 mol/mol % respectively were obtained for catalysts B, C and E. Catalysts A and D showed very high polymerization activities and also higher C-o* values. All polymers produced were characterized by SEM, DSC, extraction and molecular weight determinations [4].
References
[1] Luo Z, Li H, Li Q. Preparation of 9,9-bis(methoxymethyl)fluorene[P]. Faming Zhuanli Shenqing, 113045389, 29, 2021.
[2] Credendino R, Liguori D, Morini G, et al. Investigating phthalate and 1, 3-diether coverage and dynamics on the (104) and (110) surfaces of MgCl2-supported Ziegler–Natta catalysts[J]. The Journal of Physical Chemistr
- Related articles
- Related Qustion
- Exploring the Versatile World of 9,9-Bis(methoxymethyl)fluorene: Properties, Applications, and Handling Apr 17, 2024
In the dynamic world of chemical compounds, 9,9-Bis(methoxymethyl)fluorene stands out as a compound of increasing significance.
4-oxopiperidinium chloride is an important organic synthesis intermediate.....
Dec 6,2022Organic Synthesis IntermediateN-(2-hydroxyethyl)-N-methyl-4-toluidine is an important intermediate in organic synthesis.....
Dec 7,2022Organic Synthesis Intermediate9,9-BIS(METHOXYMETHYL)FLUORENE
182121-12-6You may like
9,9-BIS(METHOXYMETHYL)FLUORENE manufacturers
- 9,9-BIS(METHOXYMETHYL)FLUORENE
-

- $0.00 / 1g
- 2024-04-07
- CAS:182121-12-6
- Min. Order: 1g
- Purity: 99.99%
- Supply Ability: 20 tons
- 9,9-BIS(METHOXYMETHYL)FLUORENE
-

- $35.00 / 1kg
- 2024-03-27
- CAS:182121-12-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 9,9-Bis(methoxymethyl)-9H-fluorene
-

- $27.99 / 1KG
- 2022-02-25
- CAS:182121-12-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG