A FLT3 receptor tyrosine kinase inhibitor: Quizartinib dihydrochloride
Jan 23,2024
Description
Acute myeloid leukemia (AML) is a heterogeneous disease and remains a therapeutic challenge. Cytogenetics is a well-established prognostic factor. Recent discovery of molecular mutations has gained momentum, with some being potential therapeutic targets. FLT3 mutation seen in approximately one-third of the cytogenetically normal AML confers a high risk of relapse and poor survival. Quizartinib was the first drug developed specifically as an FLT3 inhibitor. It has confirmed safety and efficacy in phase I and II clinical trials and has shown survival benefits over conventional chemotherapy in patients with FLT3 ITD mutated relapsed/refractory AML[1].
Quizartinib dihydrochloride, or AC220, is a highly potent oral FLT3 receptor tyrosine kinase inhibitor approved in Japan for treating patients with relapsed/refractory FLT3-ITD-positive AML. As a second-generation inhibitor of FLT3, quizartinib has demonstrated several advantages over first-generation FLT3 inhibitors, which display less optimal tolerability, potency, and oral pharmacokinetic properties.
Synthesis method
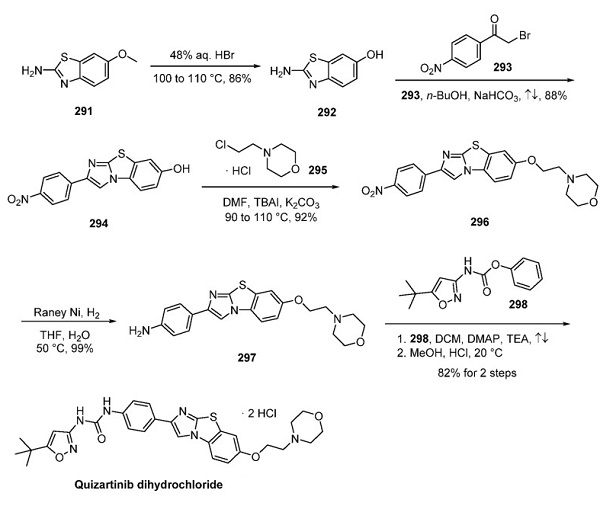
The synthetic route to quizartinib dihydrochloride has been described in several published reports by Ambit Biosciences, and this linear route is outlined above. Aminobenzothiazole 291 was demethylated using HBr to liberate phenol 292, which involved a cyclization reaction upon subjection to α-bromo ketone 293 and base to give tricyclic phenol 294. Alkylation of 294 with chloromorpholine 295 furnished 296, making way for the reduction of the nitro group to generate aniline 297. The reaction of this intermediate with carbamate 298 provided quizartinib as the free base, which was subjected to methanolic HCl to provide the bis(HCl) salt of quizartinib in an 89% yield[2].
References
[1] Kiran Naqvi, Farhad Ravandi. “FLT3 inhibitor quizartinib (AC220).” Leukemia Lymphoma 60 8 (2019): 1866–1876.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
- Related articles
- Related Qustion
?Triton X-100 is a surfactant used in a wide range of applications, including agriculture, but its effects on crops are unknown.....
Jan 23,2024SurfactantSelinexor is a first-in-class, oral, small molecule Exportin-1 (XPO1) inhibitor that is being developed by Karyopharm Therapeutics for the treatment of cancer.....
Jan 23,2024InhibitorsAC 010220 (dihydrochloride)
1132827-21-4You may like




