Dimethyl Sulfoxide (DMSO)-a widely used organosulfur compound
Dec 16,2019
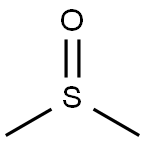
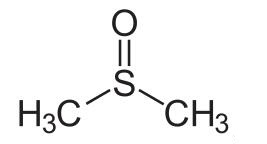
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula (CH3)2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high melting point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after contact with the skin [1]. DMSO is a 2-carbon sulfoxide in which the sulfur atom has two methyl substituents. It has a role as polar aprotic solvent, radical scavenger, non-narcotic analgesic, antidote, MRI contrast agent, Escherichia coli metabolite and alkylating agent [2]. DMSO appears as a clear liquid, essentially odorless. Closed cup flash point 192°F. Vapors are heavier than air.
In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds,[3] with a nonbonded electron pair on the approximately tetrahedral sulfur atom.
DMSO was first synthesized in 1866 by the Russian scientist Alexander Zaytsev, who reported his findings in 1867. Dimethyl sulfoxide is produced industrially from dimethyl sulfide, a by-product of the Kraft process, by oxidation with oxygen or nitrogen dioxide [3].
DMSO is a highly polar organic liquid that is used widely as a chemical solvent and a free radical scavenger. It shows a range of pharmacological activity including analgesia and anti-inflammation. Because of its ability to penetrate biological membranes, it is used as a vehicle for topical application of pharmaceuticals. It is also used to protect cells and tissue during cryopreservation and has been used to treat extravasation damage caused by anthracycline-based chemotherapy.
DMSO is a polar aprotic solvent and is less toxic than other members of this class, such as dimethylformamide, dimethylacetamide, N-methyl-2-pyrrolidone, and HMPA. DMSO is frequently used as a solvent for chemical reactions involving salts, most notably Finkelstein reactions and other nucleophilic substitutions. It is also extensively used as an extractant in biochemistry and cell biology. Because DMSO is only weakly acidic, it tolerates relatively strong bases and as such has been extensively used in the study of carbanions. A set of non-aqueous pKa values (C-H, O-H, S-H and N-H acidities) for thousands of organic compounds have been determined in DMSO solution.
DMSO is used in polymerase chain reaction (PCR) to inhibit secondary structures in the DNA template or the DNA primers. It is added to the PCR mix before reacting, where it interferes with the self-complementarity of the DNA, minimizing interfering reactions. DMSO may also be used as a cryoprotectant, added to cell media to reduce ice formation and thereby prevent cell death during the freezing process. Approximately 10% may be used with a slow-freeze method, and the cells may be frozen at −80 °C (−112 °F) or stored in liquid nitrogen safely. DMSO has been used as a co-solvent to assist absorption of the flavonol glycoside Icariin into the C. elegans nematode worm.
Use of DMSO in medicine dates from around 1963, when an Oregon Health & Science University Medical School team, headed by Stanley Jacob, discovered it could penetrate the skin and other membranes without damaging them and could carry other compounds into a biological system. In medicine, DMSO is predominantly used as a topical analgesic, a vehicle for topical application of pharmaceuticals, as an anti-inflammatory, and an antioxidant. Because DMSO increases the rate of absorption of some compounds through biological tissues, including skin, it is used in some transdermal drug delivery systems. Its effect may be enhanced with the addition of EDTA. It is frequently compounded with antifungal medications, enabling them to penetrate not just skin but also toenails and fingernails.
DMSO is a rapidly penetrating molecule that increases cells tolerance to osmotic stress induced by cryopreservation. DMSO was originally used as an anti-inflammatory reagent but was later found to cause an array of side effects when administered to patients. DMSO toxicity in HPC product infusions was shown to affect multiple organs, including respiratory, cardiovascular, gastrointestinal, hepatic, and renal systems. DMSO is also associated with intravascular hemolysis. Side effects related to infusion of thawed products are usually mild to moderate, most commonly nausea, vomiting, hypertension, or allergic reactions. Severe side effects are rare but cardiovascular, neurologic, and respiratory side effects have been reported, with some of them being severe or fatal. The rate of side effects was shown to correlate with higher DMSO volume. Current recommendation is to infuse less than 1 mL DMSO/kg recipient weight [4]. Contact with the skin may cause stinging and burning and lead to an odor of garlic on the breath. An excellent solvent that can transport toxic solutes through the skin. High vapor concentrations may cause headache, dizziness, and sedation.
References
[1] https://en.wikipedia.org/wiki/Dimethyl_sulfoxide
[2] https://pubchem.ncbi.nlm.nih.gov/compound/Dimethyl-sulfoxide
[3] Kathrin-Maria Roy "Sulfones and Sulfoxides" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_487
[4] Ljiljana V. Vasovic MD, Ronit Reich-Slotky PhD, MSc, Ruchika Goel MD, MPH, Chapter 13 - Hematopoietic Stem Cell Collections and Cellular Therapies, Clinical Principles of Transfusion Medicine 2018, Pages 151-167, https://doi.org/10.1016/B978-0-323-54458-0.00013-1
- Related articles
- Related Qustion
- Applications of Dimethyl sulfoxide in chemical and medical industry Dec 16, 2024
Dimethyl sulfoxide (DMSO) is a colourless, widely used and powerful non-protonic solvent. It is known as a universal solvent and can be miscible with water and a wide range of organic solvents.
- Dimethyl Sulfoxide: A Widely Used Polar Aprotic Solvent Dec 27, 2023
Dimethyl Sulfoxide (DMSO) is a polar aprotic molecule that has the peculiar property of having a mildly nucleophilic sulfur and a basic oxygen. It is used as a reagent in a variety of different reactions.
- Is Dimethyl sulfoxideharmful to Humans? Dec 1, 2022
DMSO can cause contaminants, toxins, and medicines to be absorbed through the skin, which may cause unexpected effects.
Punicalagin is an ellagitannin, a type of phenolic compound. It is found in forms alpha and beta in pomegranates (Punica granatum), in Terminalia catappa and Terminalia myriocarpa, and in Combretum molle, the velvet bushwillow.....
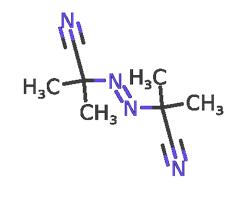
Dec 16,2019Plant extractsAzobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula [(CH3)2C(CN)]2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water.....
Dec 16,2019Organic Raw MaterialDimethyl sulfoxide
67-68-5You may like
Dimethyl sulfoxide manufacturers
- Dimethyl sulfoxide
-

- $6.00 / 1kg
- 2025-04-17
- CAS:67-68-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
- Dimethyl sulfoxide
-

- $0.00 / 1KG
- 2025-04-17
- CAS:67-68-5
- Min. Order: 1KG
- Purity: >99% GC
- Supply Ability: 1000KG
- DIMETHYLSULFOXIDE(DMSO)
-

- $0.00 / 225Kg/Drum
- 2025-04-17
- CAS:67-68-5
- Min. Order: 1000KG
- Purity: 99.8%
- Supply Ability: 18mt