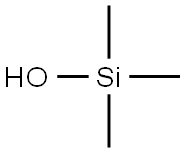Hydroxytrimethylsilane: vibrational spectra and its detection method
Feb 20,2025
Introduction
Hydroxytrimethylsilane (Trimethylsilanol, Figure 1) in industrial sectors has gained wide attention due to the widespread use of silicon materials and their detrimental effects on equipments and products [1]. Hydroxytrimethylsilane is a silanol but often is considered to belong to the siloxane group. It is the most volatile siloxane with a vapor pressure of 73.9 mmHg at 25℃ . Siloxanes are a family of silicon containing organic compounds that are widely used in manufacture of commercial and consumer products, for example, detergents, deodorants, and cosmetics. Siloxanes are considered safe to the general population and available toxicological studies target octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5), and dodecamethylcyclohexasiloxane (D6); thus, no inhalation toxicity data are available for hydroxytrimethylsilane. Limited oral and skin exposure studies show that hydroxytrimethylsilane causes nervous system depression and anesthesia at high doses. Oral toxicity tests determined a no observable effects limit of 160 mg/kg/day in rats . The U.S. Occupational Safety & Health Administration has not set exposure limits for hydroxytrimethylsilane. The U.S. National Academies have set 65 mg/m3 and 32 mg/m3 as 24-hour and long-term spacecraft maximum allowable concentrations for hydroxytrimethylsilane, respectively. The U.S. Environmental Protection Agency (EPA) is proposing a chemical action plan for siloxanes to understand human health risk associated with siloxane exposure [2].

Vibrational spectra of Hydroxytrimethylsilane
The low-frequency region of the hydroxytrimethylsilane spectrum was investigated only by Rouviere et al. . They found weak bands near 150 cm−1 both in Raman and IR spectra of liquid. These bands may be attributed to δ SiOH, but the fact that their positions do not change upon the deuteration of the hydroxyl group and these bands were not observed in the gas phase allowed the authors to attribute them to the cage vibrations of hydrogen-bonded associates . In all previous publications on the vibrational spectra of hydroxytrimethylsilane, the authors considered the large shift of ν OH in going from the gas to condensed phases, while they ascribed the δ SiOH mode in different phases to bands with close frequencies. However, in our analysis of the vibrational spectra of hydrogen-bonded associates of H3SiOH molecules it was shown that the well-known large red shift of ν OH bands upon formation of hydrogen-bonded species is accompanied by a substantial blue shift of δ SiOH.[3]
The assignment of the SiOH group vibrations of hydroxytrimethylsilane, which is still controversial, is proposed. This assignment is based on theoretical B3LYP force field scaled using the constants of the (CH3)3Si group optimized to fit experimental vibrational frequencies of (CH3)3SiF and (CD3)3SiF molecules as well as the OH stretching scale factor from methanol. The ab initio force field defined in this way gives a good agreement of the theoretical vibrational frequencies of hydroxytrimethylsilane with the positions of IR and Raman bands observed in the gas phase. This force field predicts the greatest contribution of the δ SiOH coordinates to the vibration with frequency of 804 cm−1. The elimination of the coupling of the SiOH deformation with methyl rocking modes by the normal coordinate treatment of (CD3)3SiOH gives 832 cm−1 for silanol deformation which is in a good agreement with the 834 cm−1 value proposed earlier for the bending mode of free silanol groups. The geometry and force field of the open chain H3SiOH trimer is computed to model the change of the δ SiOH frequencies upon formation of the hydrogen-bonded polymers. This model predicts a significant shift of SiOH bending frequencies to the 1000–1200 cm−1 range while those of SiOD to the 800–850 cm−1 range. These predictions allow us to ascribe the 1087 cm−1 band observed in the IR spectrum of crystalline (CH3)3SiOH and the Raman 775 cm−1 band of the liquid (CH3)3SiOD to deformations of the hydrogen-bonded silanol groups.[3]
Thermal desorption (TD)-gas chromatography (GC)-mass spectrometry (MS) method
High-sensitivity methods have not been designed specifically for airborne hydroxytrimethylsilane in industrial environments that have special needs to control hydroxytrimethylsilane, for example, the semiconductor manufacturing workshops.
Lee Jae-Hwan's study develops a thermal desorption (TD)-gas chromatography (GC)-spectrometry (MS) method for trace-level hydroxytrimethylsilane analysis in occupational indoor air. The method is optimized by comparing adsorbent configurations and analysis solvents, choosing an appropriate desorption temperature and applying a more sensitive selected ion monitoring (SIM) mode in MS. The method performance is evaluated in laboratory and in a semiconductor fabrication factory.
In Lee's study, they developed a sensitive sampling and analytical method for measuring trace levels of hydroxytrimethylsilane in indoor air of semiconductor fabrication environments. Method optimization suggested that best performance could be obtained if using dual-bed (Tenax TA followed by Carboxen 569) adsorbent configuration, n-decane as analysis solvent, and a thermal desorption temperature of 300℃. Laboratory and field evaluation revealed satisfactory performance of the methods: a reasonable recovery of 87%, typical replicate precision of within 15%, high linearity (R2 = 0.9999), and a low MDL of 2.8 ng/m3 for a 20-L sample. The hydroxytrimethylsilane on adsorbents could stay stable for up to 4 days, with a loss of 23% in 14 days and longer. hydroxytrimethylsilane concentrations varied from 1.02 to 27.30 μg/m3 in the indoor air of a semiconductor fabrication workshop. To our knowledge, these were the first measurements of indoor airborne hydroxytrimethylsilane in occupational indoor air. We also suggest the need to develop real-time monitoring techniques for the maintenance and control of the scanner lens system during lithography process in semiconductor wafer manufacturing.[2]
References
[1] Ohannessian A , Desjardin V , Chatain V ,et al. Volatile organic silicon compounds: the most undesirable contaminants in biogases[J].Water Science and Technology, 2008(9):58.DOI:10.2166/wst.2008.498.
[2] Lee JH, Jia C, Kim YD, et al. An Optimized Adsorbent Sampling Combined to Thermal Desorption GC-MS Method for hydroxytrimethylsilane in Industrial Environments. Int J Anal Chem. 2012;2012:690356. doi:10.1155/2012/690356.
[3] Ignatyev IS, Partal F, López González JJ, Sundius T. Vibrational spectra of hydroxytrimethylsilane. The problem of the assignment of the SiOH group frequencies. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60(5):1169-1178. doi:10.1016/j.saa.2003.07.004.
- Related articles
- Related Qustion
Hydrocortisone acetate, a synthetic derivative of the natural steroid hormone hydrocortisone, is widely used in the pharmaceutical and healthcare industries.....
Apr 10,2025Chemical ReagentsAsiaticoside is a pentacyclic triterpenoid saponin with a variety of pharmacological effects including antitumor, neuroprotective and wound healing effects.....
Feb 20,2025Chinese Herbshydroxytrimethylsilane
1066-40-6You may like
hydroxytrimethylsilane manufacturers
- hydroxytrimethylsilane
-

- $0.00 / 1kg
- 2025-04-11
- CAS:1066-40-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- hydroxytrimethylsilane
-

- $0.00 / 25kg
- 2025-03-21
- CAS:1066-40-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000KG/month
- Hydroxytrimethylsilane
-
- $7.98/ kg
- 2024-12-16
- CAS:1066-40-6
- Min. Order: 1kg
- Purity: 98.76%
- Supply Ability: 18000 tons per year