MECHANISMS OF FORMATION OF ACETOIN BY BACTERIA
Nov 14,2019
The formation of acetoin (acetylmethylcarbinol) by various tissues has been shown to be a synthetic process, the 4-carbon ketol originating from pyruvic acid in bacteria (1, 2), from pyruvic acid and acetaldehyde in yeast (3) and animal tissues (4), and from acetaldehyde alone in plants (5) and in animal tissues (4). The mechanism of formation of acetoin has been studied chiefly in yeast (6) and in pig heart (7), and it appears to involve the condensation of two 2-carbon units in these tissues. In studies on pyruvic acid metabolism of bacteria Watt and Krampitz (8) suggest.ed that a new intermediate, cr-acetolactic acid (CH&OH(COCHI)- COOH), probably formed by condensation of acetaldehyde and pyruvic acid, might be involved in both the synthesis of acetoin and in the anaerobic dismutation of pyruvic acid to lactic and acetic acids and carbon dioxide. Decarboxylation of oc-acetolactic acid would yield acetoin. Hydrolytic (or phosphorolytic) cleavage of or-acetolactic acid (Equation 1) could account for the formation of the products of the pyruvic acid dismutation reaction (9).
Bacterial preparations capable of performing the pyruvic acid dismutation reaction were shown to be able to fix labeled carbon dioxide into the carboxy1 group of pyruvic acid (10). These results might be explained by the reversible fixation of carbon dioxide into the carboxyl group of ar-acetolactic acid (fl-keto acid carboxylation), which subsequently formed pyruvic acid and acetaldehyde by a reversal of the synthetic reaction. Krampitz (11) has synthesized ar-acetolactic acid and shown that a bacterial preparation capable of forming acetoin from pyruvic acid is also able to decarboxylate the synthetic acid rapidly.
Methods and Materials
The crude enzyme capable of forming acetoin was prepared from Aerobatter aerogenes, as described by Silverman and We&man (l), except that the cells, after being crushed with glass, were extracted with 0.13 M potassium phosphate buffer of pH 5.5 (2 ml. per gm. of original cell paste). An enzyme preparation capable of catalyzing the pyruvic acid dismutation reaction was prepared from cells of A. aerogenes obtained by inoculating 10 liters of a medium containing 1 per cent peptone, 0.5 per cent beef extract, 0.5 per cent yeast extract, and 0.5 per cent NaCl (pH adjusted to 7.7 with NaOH) with 100 ml. of a 24 hour culture of the organism grown in the same medium. The 10 liters of culture were aerated at 37” for 19 hours and the cells were harvested with the Sharples centrifuge. The yield of cell paste was 38 gm. The enzyme preparation was obtained by crushing the cells with powdered glass (13) and extracting with 0.05 M potassium phosphate buffer, pH 7.0 (2 ml. per gm. of original cell paste). Acetone powders were prepared by suspending a washed bacterial paste in 50 volumes of cold (- 18”) acetone and incubating at this temperature for 1 hour. The acetone was decanted from the cells and the latter were dried by vacuum desiccation. Pyruvic acid, ar-ketoglutaric acid, and a-ketobutyric acid were used as the sodium salts and assayed manometrically by oxidation with ceric sulfate (14). Acetoin was determined by the micromethod of Westerfeld (15). ar-Acetolactic acid was synthesized according to the method of Krampitz (11). Cocarboxylase was kindly supplied by Merck and Company, Inc. Carbon dioxide was measured with the usual Warburg manometric apparatus. In almost all the experiments described in this report air has been used as the gas phase. It was originally observed (1) that the crude enzyme from A. aerogenes was indifferent to the gas phase employed, and this fact has been verified in our laboratory.
Results
Properties of Crude Enzymes It has been shown (8, 11, 16) that crude cell-free extracts obtained from bacteria capable of producing acetoin from pyruvic acid, according to Equation 4, are also capable of decarboxylating cr-acetolactic acid (Equation 3).
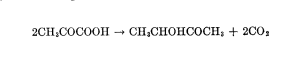
by guest on November 14, 2019 https://www.jbc.org/ Downloaded from An attempt was made to determine whether the component of the crude enzyme preparation that decarboxylated ac-acetolactic acid (ar-acetolactic acid decarboxylase) was specific for this /3-keto acid. Table I indicates that acetoacetic acid and oxalacetic acid are not attacked by the enzyme.
Enzyme Resolution
40 ml. of the crude enzyme preparation obtained from A. aerogenes were treated with 6 gm. of alumina (Alumina A-303, Aluminum Company of America) with constant stirring. After 15 minutes the mixture was centrifuged at 0”. The supernatant solution (and subsequent fractions) was assayed for the ability to decarboxylate pyruvic and a-acetolactic acids. Most of the activity of the cy-acetolactic decarboxylase remained in the supernatant solution. The alumina precipitate was washed three times with 25 ml. portions of ice-cold 0.13 M potassium phosphate buffer, pH 5.5, and the washes were discarded. The alumina precipitate was then eluted twice with 10 ml. portions of 0.13 M K2HPOd and the eluates were combined. The combined eluates were adjusted to pH 7.0 with ice-cold 1 M H3P04. To 20 ml. of eluate were added dropwise with stirring 16 ml. of cold (-18”) 95 per cent ethyl alcohol. The enzyme-alcohol solution was maintained at -3” by the use of an ice-salt bath. Precipitation was allowed to take place for 12 hours at - 18”. The mixture was then centrifuged at 20,000 X g for 15 minutes. The supernatant solution was discarded and the precipitate was triturated in 10 ml. of 0.07 M potassium phosphate buffer, pH 6.0. Only a small amount of the precipitate redissolved. The insoluble material was removed by centrifugation and the water-clear supernatant solution (alcohol-precipitable fraction) was found to contain 27.4 per cent of the original activity of the crude preparation on pyruvic acid but only 1.7 per cent of the activity of a-acetolactic acid decarboxylase.
FORMATION OF ACETOIN BY BACTERIA
It mill be noticed (Table II) that the sum of the activities of the supernatant solution from the alumina treatment and the alumina eluate fractions on cr-acetolactic acid totals 127.5 per cent compared with the corresponding activity of the crude enzyme preparation, It has been consistently found that subjecting the crude enzyme preparation to adsorption on alumina with subsequent elution results in an absolute increase of activity in the ac-acetolactic acid decarboxylase. In some cases there has been as much as 200 per cent recovery. The alumina used does not alter the activity of cu-acetolactic decarboxylase when added directly to an assay vessel and the effect, therefore, cannot be directly attributed to this material.
A preparation active for the decarboxylation of cr-acetolactic acid (o(- acetolactic decarboxylase) but completely inactive on pyruvic acid was obtained by heating the crude enzyme preparation to 70” for 2 minutes. The heat-denatured protein was removed by centrifugation and discarded.
Identification of ar-Acetolactic Acid
The action of the crude enzyme preparation with that of the alcoholprecipitable fraction on pyruvic acid is compared in Table III. The alcohol-precipitable fraction produced equal amounts of or-acetolactic acid and COz from pyruvic acid. The cr-acetolactic acid was determined by decarboxylation with strong H2S04 (i.e., 0.1 ml. of 18 N HzS04 per ml. of solution). The values observed are in accord with Equation 2. The crude enzyme preparation converted all of the pyruvic acid to acetoin and COZ according to Equation 4; virtually no p-keto acid was formed. Both the enzymatically and synthetically produced a-acetolactic acids form the osazone of diacetyl when treated with phenylhydrazine. There is a concomitant formation of an equivalent quantity of CO2 during the formation of the derivative. Sufficient a-acetolactic acid was formed enzymatically by permitting 5 ml. of the alcohol-precipitable fraction to act on 10.4 mM of sodium pyruvate for 135 minutes. The reaction was carried out in the presence of 0.25 M potassium phosphate buffer, pH 5.5, and 0.5 mg. of cocarboxylase. The osazone was formed by adding 30 ml. of phenylhydrazine reagent (4 gm. of phenylhydrazine hydrochloride and 6.4 gm. of sodium acetate dissolved in water to 30 ml.) to the reaction mixture. Crystallization of the derivative began almost immediately. After shaking for 4 hour the precipitate was filtered, mashed with hot ethyl alcohol, and recrystallized three times from dioxane. The final product had a melting point of 251-251.5” (uncorrected). The derivative formed from chemically synthesized oc-acetolactic acid had the same melting point, and no depression was observed in the mixed meRing point of these two derivatives.
- Related articles
- Related Qustion
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH.It is a colorless volatile liquid that darkens readily upon exposure to air.....
Nov 14,2019Organic ChemistrySuccinic anhydride (SA) is an useful electrolyte additive for high voltage cathodes but has also a negative impact on graphite (Gr) or Li anodes. For this reason, the Li/electrolyte and Gr/electrolyte interfaces were investigated at 20°C an....
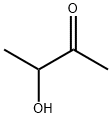
Nov 14,2019Organic ChemistryAcetoin
513-86-0You may like
- 3-Hydroxy-2-Butanone
-

- $5.00 / 1KG
- 2024-10-11
- CAS:513-86-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000kg
- Acetoin
-

- $0.00 / 25KG
- 2023-06-30
- CAS:513-86-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Acetoin
-

- $0.00 / 1kg
- 2023-06-03
- CAS:513-86-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500000kg