Synthesis of Indolizine
Jan 24,2022
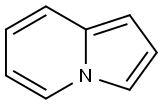
Indolizine is a bicyclic ring system compound, constituted by fusion of pyrrole and aryl rings in such a way that the nitrogen of pyrrole could be one of the bridge atoms. In other words, indolizine is a fused heterocycle, formed by overlapping of the C–N bond of electron-deficient pyridine and π-excessive pyrrole. Indolizine was first described by Angeli in 1890 but after a gap of 20 years the first synthesis of this molecule was accomplished by Scholtz and called pyrrolo[1,2-a]pyridine. The indolizine ring is widely present in nature in plants and many animals. The parent indolizine has a resonance energy in the order of 0.29 kcal/mol, which is larger than the total resonance energy of pyrrole (0.23 kcal/mol). Indolizine is best represented by a resonance hybrid of three canonical structures: I, II, and III. The electron density based on HMO calculations are in the order of 3 > 1 > 8a > 5 > 2 > 7 > 6 on the ring carbons.

Application
Numerous indolizine alkaloids in which the indolizine ring is present as either a partially or fully reduced substructural form display antiinflammatory, hypoglycemic, antiacetylcholine, central nervous system depressant, antioxidant, antibacterial, and analgesic activities. Various amino acids and hydroxy derivatives with the indolizine ring system are found useful in anticancer therapy and have also been found to display antitubercular activity. In addition to natural products, various synthetic indolizine derivatives display diverse biological activities such as anticancer, antitubercular, aromatase and phosphodiesterase inhibition, antihistaminic, and antiinflammatory.
Synthesis
The topographical survey reveals that indolizines can be synthesized in two ways.
1. The first approach is intramolecular cyclization of appropriately functionalized pyridine.
2. The other approach is by building a pyridene ring from suitably functionalized pyrrole.
1. (a) One of the simplest methods for the construction of parent indolizine199 is through intramolecular oxidative
thermal cyclization of 3-(2-pyridyl)propan-1-ol at 280°C in the presence of palladium on carbon in 50% yields.

- Related articles
- Related Qustion
Isatin was first of all reported by Erdman and Laurent in 1841 as a new product from the oxidation of indigo by nitric and chromic acids. Its utility was recognized as an intermediate for the construction of a variety of heterocycles to gen....
Jan 24,2022Organic ChemistryDigitoxin is a cardiac glycoside sometimes used in place of DIGOXIN. It has a longer half-life than digoxin; toxic effects, which are similar to those of digoxin, are longer lasting .....
Jan 24,2022Natural ProductsINDOLIZINE
274-40-8You may like