The synthesis method of Beflubutamid-M
Feb 19,2024
Description
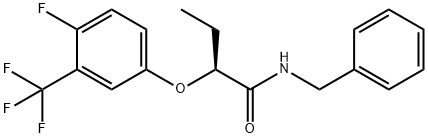
Beflubutamid-M ((-)-Beflubutamid or (S)-beflubutamid) was announced by FMC as a new herbicide in2018. It is the more active (S)-enantiomer of the established agrochemical beflubutamid (a chiral soil herbicide currently marketed as racemate against dicotyledonous weeds in cereals), which is used to control broad-leaf weeds in cereal crops. Biotests have shown that (-)-beflubutamid is at least 1000x more active than (+)-beflubutamid[1]. The mode of action of beflubutamid and its (S)-enantiomer is the inhibition of carotenoid biosynthesis by blocking phytoene desaturase, shown below.
![Butanamide, 2-[4-fluoro-3-(trifluoromethyl)phenoxy]-N-(phenylmethyl)-, (2S)- Butanamide, 2-[4-fluoro-3-(trifluoromethyl)phenoxy]-N-(phenylmethyl)-, (2S)-](/NewsImg/2024-02-19/6384393339144070875334391.png)
Synthesis method
The synthesis of beflubutamid-M is achieved with the α -aryloxycarboxylic acid 99 as a strategic intermediate. It can be prepared either by chiral resolution from its racemate using (L)-(-)- α -phenylethylamine or by enantioselective hydrogenation of α -aryloxy- α,β -unsaturated acids. Conversion of 99 with thionyl chloride affords the corresponding acid chloride, which is directly reacted with benzylamine to deliver beflubutamid-M[2].
![Butanamide, 2-[4-fluoro-3-(trifluoromethyl)phenoxy]-N-(phenylmethyl)-, (2S)- Butanamide, 2-[4-fluoro-3-(trifluoromethyl)phenoxy]-N-(phenylmethyl)-, (2S)-](/NewsImg/2024-02-19/6384393343162084354161886.png)
References
[1] Buerge, Ignaz J. , M. D. Mueller , and T. Poiger . "The Chiral Herbicide Beflubutamid (II): Enantioselective Degradation and Enantiomerization in Soil, and Formation/Degradation of Chiral Metabolites." Environmental Science & Technology 47.13(2013):6812-6818.
[2] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringCyclopyranil was announced by Kyoyu Agri as a new herbicide in 2017.....
Feb 19,2024Chemical pesticides ?