Vitamin A: Properties, Production process and Uses
Mar 13,2024
Properties of vitamin A
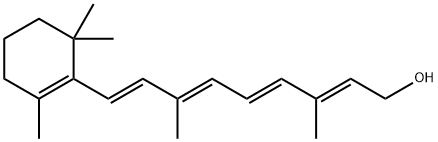
Vitamin A, also known as retinol, all-trans-retinol, all-trans-retinyl alcohol, transretinol and (all-E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen- 1-ol, is a derivative of β-ionone and acetylene.
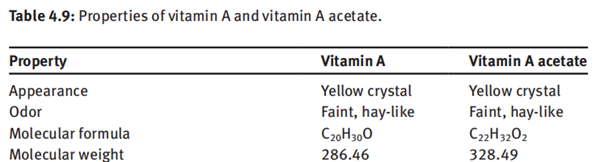
Vitamin A is a yellow flaky crystal or crystalline powder with a melting point of 62–64°C and a boiling point of 137–138°C (10−6 mmHg). It is a lipid-soluble vitamin, which is soluble in most organic solvents such as alcohols, ethers, hydrocarbons and halogenated hydrocarbons but insoluble in water and glycerin. The physical properties of vitamin A and vitamin A acetate are summarized in Table 4.9.

There are four conjugated double bonds in the side chain of vitamin A; thus, 16 geometric isomers in theory are possible. Due to the steric hindrance effect, the only geometric isomers existing in nature are the unhindered all-E, 9Z-, 13Z-, 9Z-, 13Z- and hindered 11Z-vitamin A. Only the all-E isomer has full activity of vitamin A. Vitamin A is unstable. It is easily destroyed by acid, air, oxidizing substances, high temperature and ultraviolet radiation. Since vitamin A acetate is more stable than vitamin A alcohol, the commercial form of vitamin A is mainly vitamin A acetate. Usually, the commercial product called vitamin A on the market is actually the acetate of vitamin A.
Production processes of vitamin A
Roche synthesis process (C14 + C6 route)
In Roche synthesis process, β-ionone (C14) and 3-methyl-2-penten-4-yn-1-ol (C6 alcohol) are used as starting materials, so it is also called C14 + C6 route. Figure 4.9 shows the Roche route to vitamin A acetate. β-Ionone undergoes a Darzen's condensation with methyl chloroacetate to produce the C14 aldehyde. The side chain is constructed by a Grignard reaction with C6 alcohol, resulting in the generation of diol, which is partially hydrogenated and acylated to obtain a monoacetate. Elimination and isomerization of the monoacetate give vitamin A acetate.

BASF synthesis process (C15 + C5)
A typical feature of this process is C15 + C5 route involving a Witting reaction. The reaction process is shown in Figure 4.10. β-Ionone reacts with acetylene to form ethynyl-β-ionol, followed by partial hydrogenation to obtain vinyl-β-ionol. Alternatively, ethynyl-β-ionol could be obtained by direct reaction of β-ionone with a vinyl Grignard. The vinyl-β-ionol was transformed into a C15 phosphonium salt by reaction with triphenylphosphine and HCl. The resulting C15 phosphonium salt underwent a Witting reaction with C5 aldehyde in the presence of sodium methoxide catalyst to produce vitamin A acetate.

Rhône-Poulenc route (C15 + C5) to vitamin A acetate
Rhône-Poulenc developed another C15 + C5 route using sulfone as an intermediate. Figure 4.11 represents the reaction process for vitamin A acetate by Rhône-Poulenc synthesis (C15 + C5). Vinyl-β-ionol was obtained by ethynylation and partial hydrogenation of β-ionone in the same way as BASF route. Vinyl-β-ionol reacted with sodium benzene sulfinate to produce an allylic sulfone (C15). The sulfone underwent a Julia reaction with C5 chloroacetyl to generate a C20 sulfone, followed by an elimination reaction to produce vitamin A acetate.

Wittig–Horner route (C15 + C5) to vitamin A acetate
Zhejiang Medicine Co. Ltd. has developed the Wittig–Horner route to vitamin A acetate using a phosphonate as an intermediate. The reaction process is shown in Figure 4.12. The C14 aldehyde was prepared by that same method as Roche route, and reacted with tetraethyl methylene disphosphonate in strong alkaline environment to obtain a diethyl phosphonate of 3-methyl-5-(2,6,6-trimethyl-1-cyclohexene)-1,4-pentadiene (C15). Diethyl C15 phosphonate reacted with C5 aldehyde in a strongly alkaline environment to give vitamin A acetate.

Uses of vitamin A
Vitamin A is applied as a dietary supplement and an important medicine for the treatment of deficiency syndromes. It is not an endogenously generated vitamin; therefore, it is necessary to be supplied from dietary or vitamin supplement sources. The physiological function of vitamin A is to maintain the integrity of epithelial tissue and the permeability of cell membrane. It is very important to maintain normal vision. Moreover, it is also helpful for reproduction and lactation, and for promoting the growth and development of the human body and animals. In vitamin A-deficient human body and animals, epithelial changes such as keratinization, dry, scaly skin, changes in the epidermis of the respiratory tract and susceptibility to bacterial infections can occur. There will also be insufficient rhodopsin, weak adaptability to dark light, night blindness and conjunctivitis.
- Related articles
- Related Qustion
- What are the food sources of vitamin A? Oct 15, 2024
Vitamin A may be found in foods of animal origin or may result from the metabolism of carotenoids found in plant foods.
- All-trans-retinol: Pharmacodynamics, Mechanism,Toxicity, Applications, Preparation Apr 27, 2023
All-trans-retinol, also known as vitamin A, is a fat-soluble vitamin that is essential for normal vision, immune system function, and growth and development of the human body.
- The role of vitamin A in diabetes Sep 23, 2019
There are no unnecessary surface receptors in human cells. They all serve a purpose but which, in many cases, is still unknown and because of that they are called "orphan" receptors. When we discovered that insulin cells have a cell surface
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringBoth Pyrethrin and Permethrin are insecticides, in this article, we will discuss the difference in source, property, applications and safety between them.....
Mar 13,2024Chemical pesticides ?