What is Tetramethylbenzidine?
Dec 31,2019
Tetramethylbenzidine(TMB) is a chromogenic substrate used in staining procedures in immunohistochemistry as well as being a visualising reagent used in enzyme-linked immunosorbent assays (ELISA). It is a white solid that forms a pale blue-green liquid in solution with ethyl acetate. This product is degraded by sunlight and by fluorescent lights[1].
Tetramethylbenzidine can act as a hydrogen donor for the reduction of hydrogen peroxide to water by peroxidase enzymes such as horseradish peroxidase. The resulting diimine causes the solution to take on a blue colour, and this colour change can be read on a spectrophotometer at the wavelengths of 370 and 650 nm. The reaction can be halted by addition of acid or another stop reagent. Using sulfuric acid turns Tetramethylbenzidine yellow. The colour may be read at 450 nm.
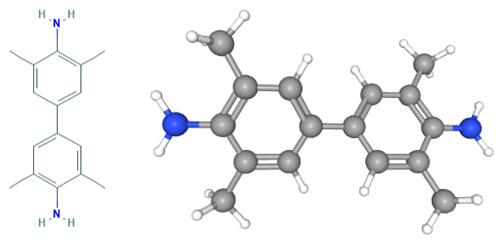
Fig 1. Chemical structure formula and three-dimensional structure of Tetramethylbenzidine
Tetramethylbenzidine should be kept out of direct sunlight as it is photosensitive. It is not known if Tetramethylbenzidine is carcinogenic and the evidence is contradictory: Tetramethylbenzidine is not mutagenic by the Ames test, and did not induce formation of tumors in a single-arm study of 24 rats. On that evidence, it has been used as a replacement for carcinogenic compounds such as benzidine and o-phenylenediamine[2-4].
Tetramethylbenzidine is sensitive to prolonged exposure to light. Neutralizes acids in exothermic reactions to form salts plus water. It may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.Tetramethylbenzidine is a soluble chromogen substrate for horseradish peroxidase detection systems. Tetramethylbenzidine is recommended for ELISA procedures.
Tetramethylbenzidine's production and use as a clinical reagent for testing blood may result in its release to the environment through various waste streams. If released to air, an estimated vapor pressure of 5×10-7 mm Hg at 25℃ indicates tetramethylbenzidine will exist in both the vapor and particulate phases in the ambient atmosphere. Vapor-phase will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 5 hours. Tetramethylbenzidine absorbs light greater than 290 nm, and it may be susceptible to direct photolysis in the environment; however, the rate of this potential reaction is unknown. Particulate-phase tetramethylbenzidine will be removed from the atmosphere by wet and dry deposition. If released to soil, tetramethylbenzidine is expected to have slight mobility based upon an estimated Koc of 4,000. The first pKa is estimated as 4.2, which indicates that this compound will partially exist in the protonated form under acidic conditions and cations have greater adsorption to soils than neutral molecules. Furthermore, tetramethylbenzidine is an aromatic amine which may form covalent bonds with humic materials resulting in relatively immobile quinone-like complexes. Volatilization from moist soil surfaces is not expected to be an important fate process for tetramethylbenzidine because cations do not volatilize, and the estimated Henry's Law constant of the neutral species is 7.7×10-11 atm-cu m/mole. Tetramethylbenzidine is not expected to volatilize from dry soil surfaces based upon its estimated vapor pressure.
No data regarding the biodegradation of this product in soil or natural water were found; however, a screening study on a similar compound, 3,3'-dimethylbenzidine, using sewage sludge inoculum, suggests biodegradation will occur slowly in the environment. Benzidine-based compounds such as tetramethylbenzidine are rapidly oxidized by Fe(III) and several other cations which are frequently found in environmental waters and soil. If released into water, tetramethylbenzidine is expected to adsorb to suspended solids and sediment based upon the estimated Koc. Volatilization from water surfaces is not expected to be an important fate process for either the free base or its conjugate acid based upon this compound's estimated Henry's Law constant and the fact that cations are non-volatile. An estimated BCF of 290, suggests the potential for bioconcentration in aquatic organisms is high.Tetramethylbenzidine is not expected to undergo hydrolysis due to a lack of hydrolyzable functional groups. Occupational exposure to tetramethylbenzidine may occur through inhalation and dermal contact with this compound at workplaces where this product is produced or used.
References
[1] Martin TL; Mufson EJ; Mesulam MM (1984). "The light side of horseradish peroxidase histochemistry". J Histochem Cytochem. 32 (7): 793.
[2] Ashby J; Paton D; Lefevre PA; Styles JA; Rose FL (1982). "Evaluation of two suggested methods of deactivating organic carcinogens by molecular modification". Carcinogenesis. 3 (11): 1277–1282.
[3] Chung K-T; Chen S-C; Wong TY; Li YS; Wei CI; Chou MW (2000). "Mutagenicity studies of benzidine and its analogs: Structure-activity relationships". Toxicol Sci. 56 (2): 351–356.
[4] Yang J; Wang H; Zhang H (2008). "One-pot synthesis of silver nanoplates and charge-transfer complex nanofibers". J Phys Chem C. 112 (34): 13065–13069.
- Related articles
- Related Qustion
- Tetramethylbenzidine: An Acoustogenic Photoacoustic Probe for Reactive Oxygen Species Detection Feb 19, 2024
Tetramethylbenzidine can be used in a unique, non-toxic photoacoustic probe for ROS/RNS detection, revolutionizing analytical and biological imaging applications.
- Tetramethylbenzidine: properties and applications Jul 27, 2023
Tetramethylbenzidine is a versatile compound used for color development and detection in various laboratory applications.
Tris (2,2’-bipyridyl) dichlororuthenium hexahydrate (RTDP, Ru (BPY) 3) is an oxygen-sensitive fluorescent stain. It can be used to measure the oxygen concentration of microfluidic chips.....
Dec 31,2019Organometallic compoundsBenzofuran-6-carboxylic Acid is a reagent in the development of potent LFA-1/ICAM antagonist SAR 118 as an opthalmic solution for treating dry eyes.....
Dec 31,2019Organic reagentsTetramethylbenzidine
54827-17-7You may like
Tetramethylbenzidine manufacturers
- Tetramethylbenzidine
-

- $0.00 / 1KG
- 2025-04-25
- CAS:54827-17-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 3500kg/month
- Tetramethylbenzidine
-

- $10.00 / 1KG
- 2025-04-23
- CAS:54827-17-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Tetramethylbenzidine
-

- $999.00/ kg
- 2025-04-23
- CAS:54827-17-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000