What is Tris(dibenzylideneacetone)dipalladium(PD2DBA3)
Feb 24,2021
Product Name: Tris(dibenzylideneacetone)dipalladium
Molecular Weight: 915.7174
Synonyms: Pd_2 (dba) _3; E (1, 4 e) - 1, 5 - diphenylpenta - 1, 4 - dien - 3 - one - palladium (3:2).E (1, 4 e) - 1, 5 - diphenylpenta - 1, 4 - dien - 3 - one; Tris (dibenzylideneacetone) dipalladium; Tris (dibenzylideneacetone) - dipalladium (O); Tris (dibenzylideneacetone) palladium (0); Z (1, 4 e) - 1, 5 - diphenylpenta - 1, 4 - dien - 3 - one; Bis (dibenzylideneacetone) dipalladium
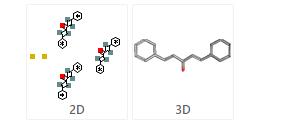
Structure
Appearance: Violet black powder

Assay: ≥97%
Packing: According to customers’ requirement
InChI: InChI = 1/3 c17h14o. 2 pd/c3 * 18-17 (13-11-15-13-11-15-13-11-15) 13-11-15-9-5-2-9-5-2;;1-14 h/h3 *;;+ / bl3 11-12 of 14 +;2 * - 11-13, 14 and 12 +;;
General Description
Tris(dibenzylideneacetone)dipalladium, CAS 60748-47-2, is insoluble in water, slightly soluble in dichloromethane and benzene. It is stable in the air, but its solution can be slowly decomposed. Tris(dibenzylideneacetone)dipalladium can be applied in coupling reaction, such as Suzuki, Kumada, Negishi, Buchwald and so on. Besides, Tris(dibenzylideneacetone)dipalladium, has certain irritations, so operators are recommended to wear suitable protective clothing, gloves and eyeshields. This product should be kept in cool and dry conditions, avoiding strong light.
Application as a Reagent
Tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) participates in the synthesis of azepane. Crystal structure of Pd2(dba)3 has been determined by three-dimensional X-ray data. Crystals of Pd2(dba)3 are reported to crystalize in triclinic system. It is widely used Pd(0) source in Pd-mediated transformations.
• Catalyst for Suzuki coupling of aryl chlorides (eq. 1)
• Catalyst for Heck coupling of aryl chlorides (eq. 2)
• Catalyst for arylation of ketones (eq. 3)
• Catalyst for Buchwald-Hartwig amination of aryl halides (eq. 4)
• Catalyst for fluorination of allylic chlorides (eq. 5)
• Catalyst for β-arylation of carboxylic esters (eq. 6)
• Catalyst for carbonylation of 1,1-dichloro-1-alkenes (eq. 7)
• Catalyst for conversion of aryl and vinyl triflates to aryl and vinyl halides (eq. 8)
Hazards
GHS Hazard Statements
H317 (95.45%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H411 (94.7%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
ECHA C&L Notifications Summary
Aggregated GHS information provided by 133 companies from 9 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
- Related articles
- Related Qustion
Methyl tert-butyl ether is an ether having methyl and tert-butyl as the two alkyl components. It has a role as a non-polar solvent, a fuel additive and a metabolite. Methyl tert-butyl ether appears as a colorless liquid with a distinctive a....
Feb 23,2021Organic ChemistryTetrakis(triphenylphosphine)palladium(0) is widely used as a catalyst for palladium-catalyzed coupling reactions.Prominent applications include the Heck reaction, Suzuki coupling, Stille coupling, Sonogashira coupling, and Negishi coupling.....
Feb 26,2021Organometallic compoundsTris(dibenzylideneacetone)dipalladium
60748-47-2You may like
Tris(dibenzylideneacetone)dipalladium manufacturers
- Tris(dibenzylideneacetone)dipalladium
-

- $100.00 / 1KG
- 2025-09-25
- CAS:60748-47-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Tris(dibenzylideneacetone)dipalladium
-

- $1.10 / 1g
- 2022-07-25
- CAS:60748-47-2
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons Min
- Tris(dibenzylideneacetone)dipalladium
-

- $20.00 / 1KG
- 2020-11-16
- CAS:60748-47-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000KG